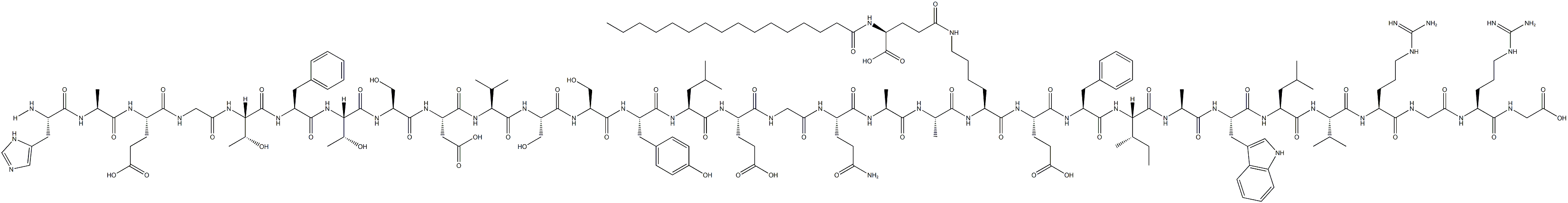
Approaches to treating type 2 diabetes mellitus (T2DM), a disease marked by islet cell dysfunction and insulin resistance, involve the use of secretagogues, which boost insulin secretion by the pancreas, sensitizers, which increase target organ sensitivity to insulin, and agents that slow down glucose absorption from the gastrointestinal tract. Liraglutide, a GLP-1 receptor agonist, has a 97% homology to GLP-1, featuring two amino acid changes and an added fatty acid side chain. The lysine in position 34 is replaced with arginine, and the lysine in position 26 is modified with a C16 acyl chain via a glutamoyl spacer.
Liraglutide's resistance to DPP-4 degradation stems from its tendency to form micelles and bind to albumin. As a once-daily treatment, it is more convenient than its predecessor, exenatide, which necessitates two daily subcutaneous injections. Liraglutide can be used alongside metformin or a sulfonylurea in patients with inadequate glycemic control on either monotherapy or combined dual therapy. It is also approved for use in combination with metformin and a thiazolidinedione for patients with insufficient glycemic control on dual therapy. Liraglutide exhibits a binding potency of 61 pM (EC50= 55 pM for GLP-1) for the cloned human GLP-1 receptor.