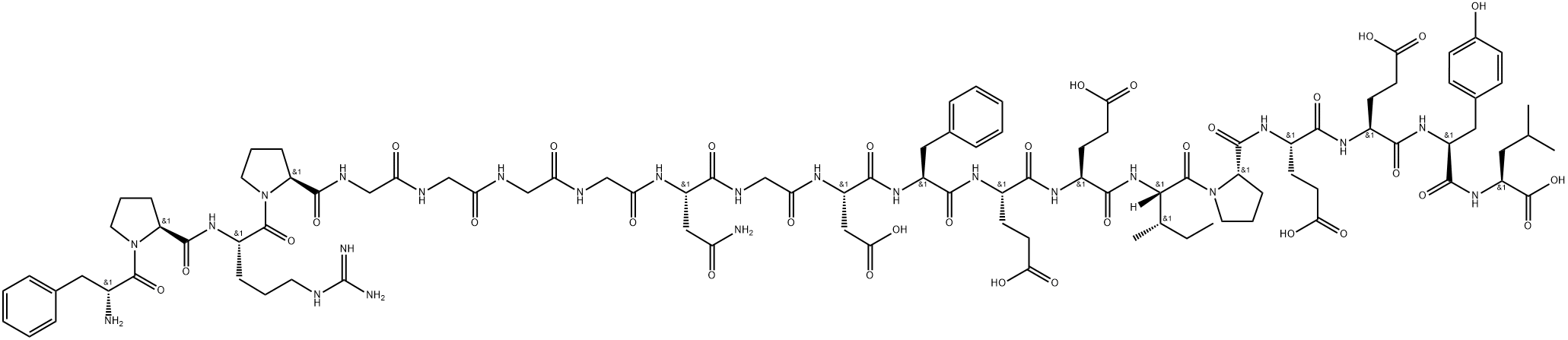
Bivalirudin is an inhibitor of α- and ζ-thrombin (Kis = 2.56 and 1.84 nM, respectively), enzymes that exhibit high fibrinogen-clotting activities. It is selective for α- and ζ-thrombin, lacking activity at trypsin and γ-thrombin, which lacks clotting activity, at a >1,000-fold excess of bivalirudin. Bivalirudin inhibits α-thrombin-stimulated activation of the clotting factors Factor X, Factor V, and prothrombin in contact-activated plasma at a concentration of 0.1 μM. Administration of bivalirudin (0.5-1.5 mg/kg, i.v.) reduces platelet deposition in a rat carotid endarterectomy model in a dose-dependent manner. Formulations containing bivalirudin have been used to prevent ischemic events during angioplasty for thrombus-containing lesions.