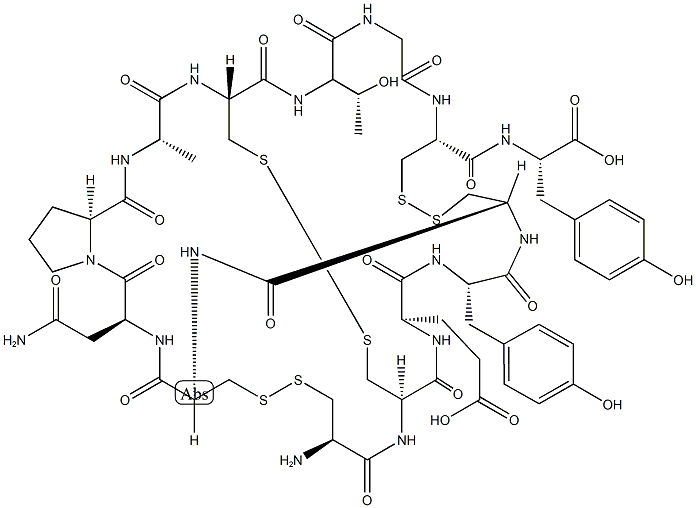
In August 2012, the US FDA approved linaclotide (also referred to as
MD-1100), a first-in-class, orally administered 14-amino acid peptide as a
therapy for patients suffering from chronic idiopathic constipation (CIC) and irritable bowel syndrome with constipation (IBS-C).
Linaclotide and its active metabolite MM-419447, which results from the
cleavage of the C-terminal tyrosine residue by carboxypeptidase A, mimic
the actions of the endogenous intestinal peptides guanylin (15 amino acids) and uroguanylin (16 amino acids) by activating guanylyl cyclase C (GC-C) on the intestinal epithelium. Activation of GC-C leads to increased intra- and extracellular levels of cGMP and activation of the CFTR ion channel, resulting in increased levels of HCO3-, Cl-, and water in the intestinal lumen and accelerated gastrointestinal transit. Based on an in vitro assaymeasuring the accumulation of cGMP in T84 cell exposed to an agonist, the EC50 of linaclotide at pH 7.0 was 99±17.5 nM. In preclinical studies in mice using the transit of activated charcoal as ameasure of efficacy, linaclotide at 100 μg/kg significantly accelerated transit compared to wild-type mice treated with charcoal only or
GC-Cnullmice treatedwith and without linaclotide.118 Efficacy was also seen
in rats treatedwith linaclotide atdoses of 5, 10, and 20 μg/kg. Linaclotide has been synthesized using conventional solid-phase peptide technology.