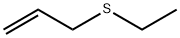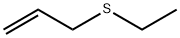The lithiated derivative can react with electrophiles at either the - or - postions. It was found that conversion of the organolithium to an organotitanium reagent (by reaction with Ti(O-iPr)4), followed by reaction with e.g. cyclohexanecarboxaldehyde, resulted in regioselective reaction at the -position to give the corresonding hydroxy sulfide.