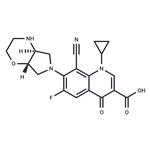
Finafloxacin, an antimicrobial agent of the 8-cyano subclass of
fluoroquinolones, was approved by the US FDA in December
2014 for treatment of acute otitis externa, commonly known as
swimmer’s ear, caused by susceptible strains of Pseudomonas
aeruginosa and Staphylococcus aureus. Finafloxacin was
developed by MerLion Pharmaceuticals in partnership with Bayer
Health Care Pharmaceuticals, and the drug was licensed by Mer-
Lion to Alcon (a division of Novartis) for development and commercialization
for ear infections in North America. In
contrast to other marketed fluoroquinolones, which display
reduced activity in slightly acidic environments, finafloxacin exhibits
increased antibacterial activity at pH 5–6, with minimum inhibitory
concentration values that are 4- to 8-fold lower than at
neutral pH. It is highly selective for bacterial type II topoisomerases,
which are involved in bacterial DNA replication, transcription,
repair, and recombination, and has broad spectrum
antibacterial activity against Gram-positive and Gram-negative
strains, including ciprofloxacin-resistant strains.
![3-Quinolinecarboxylic acid, 8-cyano-1-cyclopropyl-6-fluoro-7-[(4aS,7aS)-hexahydropyrrolo[3,4-b]-1,4-oxazin-6(2H)-yl]-1,4-dihydro-4-oxo-](https://www.chemicalbook.com/CAS/GIF/209342-40-5.gif)