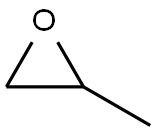
-
外観
無色澄明の液体
-
溶解性
エタノール及びアセトンに極めて溶けやすく、水に溶ける。
-
解説
1,2-epoxypropane.C3H6O(58.08).プロピレンオキシドは,酸化プロピレン,メチルオキシラン(methyloxirane)ともいう.実験室的には,2-クロロプロパノールをアルカリで処理すると得られる.工業的には,従来よりプロペンと次亜塩素酸との反応で得られるプロピレンクロロヒドリンの脱塩化水素によって製造されている.近年では,プロペンを各種ヒドロペルオキシドにより酸化する方法(ハルコン法)が開発されている.ハルコン法でエチルベンゼンヒドロペルオキシドを用いると,プロピレンオキシドとメチルベンジルアルコールが生成し,後者を酸触媒を用いて脱水するとスチレンが併産される.ほかにtert-ブチルペルオキシドを用い,イソブテンを併産する方法がある.最近,チタノシリケートを触媒に用い,液相で過酸化水素によりプロペンを酸化する直接酸化法が工業化されている.光学異性体をもつ.エーテル臭を有する引火性の無色の液体.融点-112 ℃,沸点33.9 ℃.d2525 0.826.n25D 1.363.水および有機溶剤に可溶.溶解力がとくにすぐれており,合成および天然の樹脂,各種有機物質の低沸点溶剤に用いられる.また,プロパンジオールをはじめ,界面活性剤,合成樹脂,溶剤などの合成原料となる.有毒.森北出版「化学辞典(第2版)
-
用途
中間体、殺菌剤
-
用途
プロピレングリコール、プロピレングリコールエーテルの原料 (ULLMANNS(E) (6TH, 2003)) ポリウレタン用ポリエーテル、潤滑油、界面活性剤、油水分離剤 (HSDB (2010))
-
効能
製剤補助
-
農薬用途
燻蒸剤
-
説明
Propylene oxide is an allergic and irritant agent, used
as a solvent and raw material in the chemical industry
as starting material and intermediate for a broad
spectrum of polymers.
-
化学的特性
Propylene oxide is soluble in water and miscible with most organic solvents. It is found to be an excellent low-boiling solvent for cellulose acetate, nitrocellulose, adhesive compositions and vinyl chloride-acetate resins. It is also a solvent for hydrocarbons, gums and shellac. Some of its uses are as a solvent and stabilizer in DDT aerosol-type insecticides, and as a fumigant and food preservative. Since it is an acid acceptor, it is also used as a stabilizer for vinyl chloride resins and other chlorinated systems.
-
物理的性質
Propylene oxide is a colorless liquid with an agreeable, ether-like odor. Experimentally determined detection and recognition odor threshold concentrations were 24 mg/m3 (10 ppmv) and 84 μg/m3 (35 ppmv), respectively (Hellman and Small, 1974).
-
使用
Propylene oxide is used as a fumigant forfoodstuffs; as a stabilizer for fuels, heat-ing oils, and chlorinated hydrocarbons; asa fuel–air explosive in munitions; and toenhance the decay resistance of wood andparticleboard (Mallari et al. 1989). Recentstudies indicate that the fumigant potentialof propylene oxide enhances at a low pres-sure of 100 mm Hg which could render it asan alternative to methyl bromide for rapiddisinfection of commodities (Isikber et al.2004).
-
調製方法
Propylene oxide is synthesized commercially from propylene
through the intermediate propylene chlorohydrin. It also can
be made by peroxidation of propylene using alkylhydroperoxides,
but this method produces coproducts as well, often
styrene or cumene. Propylene oxide is also synthesized via
oxidation of propylene with hydrogen peroxide, which produceswater
as the only coproduct.
-
定義
ChEBI: An epoxide that is oxirane substituted by a methyl group at position 2.
-
安全性
取扱面
プロピレンオキシドは爆発限界が2.8~37%と広く、蒸気密度は2.00と空気の2倍です。沸点も常温付近であるため非常に揮発性が高く、かつ可燃性の液化ガスで、蒸気は単独でも電気火花などにより爆発します。
空気と混合した場合は、爆発性混合ガスとなります。火気厳禁であるとともに、酸及びアルカリとの接触も避ける必要があります。また、通常の条件では、比較的安定性が高いとされていますが、高温と直射日光は避けた方が無難です。
消防法では、第4類の中でも特に引火性の高い特殊引火物に該当します。また、PRTR法においても、第一種指定化学物質に指定されています。
毒性
直接皮膚に付着すると火傷を起こし、目に入った場合は角膜炎を起こします。濃厚な蒸気を吸入した場合は、鼻、喉、気管支を強く刺激します。変異原性が認められた化学物質にも該当するため、取扱う際は、保護眼鏡、防護手袋、有機ガス用防毒マスクの着用を心がけてください。
参考文献
-
一般的な説明
A clear colorless volatile liquid with an ethereal odor. Flash point -35°F. Boiling point 95°F. Density 6.9 lb./gal. Flammable over a wide range of vapor-air concentrations. If contaminated, may polymerize with evolution of heat and possible rupture of container. Vapors irritate eyes, skin, and respiratory system. Prolonged contact with skin may result in delayed burns. Vapors heavier than air. Used as a fumigant, in making detergents and lubricants, and to make other chemicals.
-
空気と水の反応
Highly flammable. Soluble in water.
-
反応プロフィール
1,3-Propylene oxide react with oxidizing agents and strong acids . Reacts with Grignard reagents and organolithium compounds. An explosion occurred when Propylene oxide was added to an epoxy resin. Propylene oxide was concluded that polymerization was catalyzed by an amine accelerator in the resin [Bretherick, 5th Ed., 1995]. Underwent polymerization when mixed with sodium hydroxide causing ignition and explosion of a drum of the crude product. [Combust Sci. Technol., 1983].
-
危険性
Highly flammable, dangerous fire risk,
explosive limits in air 2–22%. An irritant. TLV: 20
ppm; animal carcinogen.
-
健康ハザード
Exposure to propylene oxide vapors cancause moderate to severe irritation of the eyes, mucous membranes, and skin. Inhala-tion can also produce weakness and drowsi-ness. Symptoms of acute exposure in testanimals were lachrymation, salivation, gasp-ing, and labored breathing and dischargefrom nose. Harris et al. (1989) in a studyon Fischer-344 female rats found no adverseeffect below a 300-ppm exposure level.However, at the chronic inhalation levelof 500 ppm the gain in the maternal bodyweight and food consumption were reducedsignificantly. In a similar chronic inhalationstudy on Wistar rats, Kuper et al. (1988)observed a decrease in the body weight anddegenerative and hyperplastic change in thenasal mucosa when rats were exposed to300 ppm of propylene oxide. The investiga-tors have reported an increase in the inci-dence of malignant tumors in the mammaryglands and other sites in female rats.
Contact with its dilute aqueous solutionscan produce edema, blistering, and burns onthe skin. It is mutagenic in the Ames test anda suspected animal carcinogen. Its carcino-genicity in humans is not established. Omuraet al. (1994) reported dose-dependent testicu-lar toxicity of this compound in rats inducedfrom repeated intraperitoneal injections. Itsodor threshold is 200 ppm.
-
火災危険
Vapor is heavier than air and may travel considerable distance to source of ignition and flash back. Vapors form explosive mixture with air. If polymerization takes place in container, there may be a violent rupture of container. Explosion hazard is severe when exposed to flame. Violently reacts with acetylide- forming metals such as copper or copper alloys, ammonium hydroxide; chlorosulfonic acid; hydrochloric acid; hydrofluoric acid; nitric acid; oleum and sulfuric acid. Hazardous polymerization may occur. Avoid active catalytic surfaces such as anhydrous chlorides of iron, tin, and aluminum; peroxides of iron and aluminum; and alkali metal hydroxides, high temperatures; alkalies; aqueous acids; amines and acidic alcohols.
-
使用用途
プロピレンオキシドは、そのままの状態で使用することはほとんどありません。一般的には、プロピレングリコール、ポリプロピレングリコール、顔料、医薬品中間体、殺菌剤などの原料として使用される場合が多いです。
その中でもプロピレングリコールは、プロピレンオキシドを加水分解することによって得られる物質で、大部分はの原料となります。また、ポリプロピレングリコールはプロピレンオキシドを開環重合させたポリエーテルで用の原料になります。
その他にも、適度な親水性を持つことから、食料品や化粧品などの保水剤としても広く利用されています。
-
化学反応性
Reactivity with Water No reaction; Reactivity with Common Materials: No reactions; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Polymerization can occur when this product is exposed to high temperatures or is contaminated with alkalies, aqueous acids, amines, and acidic alcohols; Inhibitor of Polymerization: Not pertinent.
-
工業用途
Propylene oxide finds its largest use as
chemical intermediates. It reacts readily with dilute amounts of mineral
acids (e.g., hydrochloric acid) to form the chlorohydrin addition product. This
reactivity with acid makes this epoxy solvent valuable acid acceptor-type
stabilizers for several chlorinated solvents. Trace amounts of hydrogen chloride
from chlorinated solvent degradation are immediately neutralized by reaction with
the propylene oxide stabilizer. Reaction of propylene oxide with
an alcohol or phenol in the presence of an acid catalyst yields the monoether of
propylene glycol.
-
接触アレルゲン
Propylene oxide is an allergic and irritant agent, used
as a solvent and raw material in the chemical industry,
as the starting material and intermediate for a broad
spectrum of polymers. It can be used as a dehydrating
agent for the preparation of slides in electron microscopy.
Occupational dermatitis was also reported following
the use of a skin disinfectant swab.
-
安全性プロファイル
Confirmed carcinogen
with experimental carcinogenic,
neoplastigenic, and tumorigenic data. Poison
by intraperitoneal route. Moderately toxic by
ingestion, inhalation, and skin contact. An
experimental teratogen. Experimental
reproductive effects. Human mutation data
reported. A severe skin and eye irritant.
Flammable liquid. A very dangerous fire and
explosion hazard when exposed to heat or
flame. Explosive reaction with epoxy resin
and sodium hydroxide. Forms explosive
mixtures with oxygen. Reacts with ethylene
oxide + polyhydric alcohol to form the
thermally unstable polyether alcohol.
Incompatible with NH4OH, chlorosulfonic
acid, HCl, HF, HNO3, oleum, H2SO4.
Dangerous; can react vigorously with
oxidizing materials. Keep away from heat
and open flame. To fight fire, use alcohol
foam, CO2, dry chemical. When heated to
decomposition it emits acrid smoke and
fumes.
-
職業ばく露
Propylene oxide is used as an interme-
diate in the production of polyether polyols and propylene
glycol; as a fumigant; in the production of adducts as ure-
thane foam ingredients; in detergent manufacture; as a
component in brake fluids.
-
発がん性
Propylene oxide is reasonably anticipated to be a human carcinogenbased on sufficient evidence of carcinogenicity from studies in experimental animals.
-
特徴
プロピレンオキシドの分子式はC3H6Oで、分子量58.08、無色の揮発性液体でエーテル臭をもつ有機化合物です。20℃における比重は0.8304、引火点-37℃、沸点33.9℃、凝固点-104.4℃、粘度は0.38mPa・s (20℃) 、水、アルコール、エーテルに溶けます。
環状エーテル構造を持ち、開環重合することでポリエーテルであるポリエチレンオキシドを作ります。一般的に活性水素化合物と容易に反応します。
-
製造方法
工業的な製造方法としては、クロロヒドリン法と直接酸化法の2つがあります。
クロロヒドリン法
プロピレンからクロロヒドリンを合成した後、プロピレンオキサイドを生成する反応です。
【クロロヒドリンの生成反応 (収率は約90%)】
CH3CH=CH2 + Cl2 + H2O → CH3CHOHCH2Cl (α-クロロヒドリン) + HCl
CH3CH=CH2 + Cl2 + H2O → CH3CHClCH2OH (β-クロロヒドリン) + HCl
【クロロヒドリンからプロピレンオキサイドの合成】
CH3CHOHCH2ClまたはCH3CHClCH2OH + 1/2Ca(OH)2
→CH3CHCH2O (プロピレンオキシド) + 1/2CaCl2 + H2O
直接酸化法
プロピレンを酸化して直接プロピレンオキシドを生成する反応です。
【プロピレンの直接酸化】
CH3CH=CH2 + 1/2O2 → CH3CHCH2O (プロピレンオキシド)
酸化剤である過酸化物により酸化するもので、なかでもイソブタン、の過酸化物を用いたものは、プロピレンオキシドだけでなく、副生物としてイソブチレン、といった化合物も同時に生成するため、工業的に有利なプロセスとしてハルコン法という名前で知られています。
-
環境運命予測
Biological. Bridié et al. (1979) reported BOD and COD values of 0.17 and 1.77 g/g using
filtered effluent from a biological sanitary waste treatment plant. These values were determined
using a standard dilution method at 20 °C for a 5 d period. When a sewage seed was used in a
separate screening test, a BOD value of 0.20 g/g was obtained. The ThOD for propylene oxide is
2.21 g/g.
Photolytic. Anticipated products from the reaction of propylene oxide with ozone or OH radicals
in the atmosphere are formaldehyde, pyruvic acid, CH3C(O)OCHO, and HC(O)OCHO
(Cupitt, 1980). An experimentally determined reaction rate constant of 5.2 x 10-13
cm3/molecule?sec was reported for the gas phase reaction of propylene oxide with OH radicals
(Güsten et al., 1981).
Chemical/Physical. The reported hydrolysis half-life for the conversion of propylene oxide to
1,2-propanediol in water at 25 °C and pH 7 is 14.6 d (Mabey and Mill, 1978). The second-order
hydrolysis rate constant of propylene oxide in 3.98 mM perchloric acid and 36.3 °C is 0.124/M?sec
(Kirkovsky et al., 1998).
May polymerize at high temperatures or on contact with alkalies, aqueous acids, amines, and
acid alcohols (NIOSH, 1997).
At an influent concentration of 1.0 g/L, treatment with GAC resulted in an effluent
concentration of 739 mg/L. The adsorbability of the GAC used was 52 mg/g carbon (Guisti et al.,
1974).
-
貯蔵
Propylene oxide is stored in a flammableliquid cabinet isolated from combustible andoxidizable materials. It is shipped in glassbottles and metal containers under a nitrogenatmosphere.
-
合成方法
プロピレンを異性化させる方法、Pd触媒存在下プロピレンと酢酸との反応による方法、塩化アリルの加水分解やアクロレインの還元による方法
-
輸送方法
UN1280 Propylene oxide, Hazard Class: 3;
Labels: 3-Flammable liquid
-
純化方法
Dry the oxide with Na2SO4 or CaH2 and fractionally distil it through a packed column (glass helices), after refluxing with Na, CaH2, or KOH pellets. [Beilstein 17 I 4, 17 II 131, 17 III/IV 17, 17/1 V 17.] The R(+)enantiomer [15448-47-2] and the S(-)enantiomer [16088-62-3] have b 33-34o/atm and [�] 20 ±14.6o (neat). [Beilstein 17/1 V 17.]
-
不和合性
Vapors may form explosive mixture with
air. Reacts with strong oxidizers, anhydrous metal chlor-
ides; chlorine, iron, strong acids; caustics and peroxides.
Polymerization may occur due to high temperatures or con-
tamination with alkalis, aqueous acids; amines, metal chlor-
ides; and acidic alcohols. Attacks some plastics, coatings
and rubber.
-
廃棄物の処理
Concentrated waste contain-
ing no peroxides-discharge liquid at a controlled rate near a
pilot flame. Concentrated waste containing peroxides-
perforation of a container of the waste from a safe distance
followed by open burning