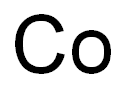-
外観
灰色, 粉末又は塊
-
性質
銀白色の金属で、見かけは鉄、ニッケルに似ている。α(アルファ)形(六方最密充填(じゅうてん))、β(ベータ)形(立方最密充填)の二つの結晶変態がある。常温ではα形のほうが安定であるが、417℃以上でβ形に変わる。しかし、両形のエネルギー差が少ないため、冷却によって全部の原子層が六方型に戻らず、部分的に立方型が残る。このような充填層の乱れは塑性変形によっても生ずる。いずれの変態も強磁性を示すが、1121℃で常磁性に変わる。鉄、ニッケル類似の展延性をもつが、粘度は錬鉄より大きく、硬さと剛性は鋼よりも大きい。
化学的安定性は状態によって異なる。水素還元法で製造した微粉状のものは、構造的にはβ形であるが、空気中で熱すると火を発して燃え、不純なものは自然発火する。これに対し粒状のものは、常温で空気中に長時間放置しても酸化物の被膜が生ずるだけで、著しい変化はおこらない。熱すると酸化が進み、白熱すれば燃えて四酸化三コバルトCo3O4を生ずる。塩酸や希硫酸には鉄よりは溶けにくい。希硝酸にはよく溶けるが、濃硝酸に対しては鉄と同様に不動態をつくるので溶けない。カ性アルカリには鉄、ニッケルと同様不溶であるが、アンモニアを含む(とくに空気や酸化剤が共存する)場合には錯塩をつくって溶ける。コバルトは酸化数ⅡおよびⅢの状態で多くの化合物をつくる。とくにコバルト(Ⅲ)は多くの安定な錯塩(多くは6配位型)をつくることで有名であり、錯塩化学の基礎的研究の多くはこの種の化合物で行われている。
-
存在
1735年、スウェーデンのブラントGeorg Brandt(1694―1768)が鉱石から元素を単体として取り出すことに成功し、以来、元素そのものがコバルトとよばれるようになった。
コバルトは砒(ひ)化鉱、硫化鉱、酸化鉱として岩石圏に広く分布しているが、そのほとんどがニッケル、銅などの他の金属を伴っている。おもなものとしてスマルタイトCoAs2、コバルタイトCoAsS、コバルト華3CoO・As2O5・8H2O、呉須土(ごすど)などがあげられる。一方、ニッケル、銅、鉛などの鉱石で少量のコバルトを含んでいるものも多く、工業資源としてはむしろこのほうが重要である。隕鉄(いんてつ)の中にも遊離状態で平均0.6%程度存在する。また、きわめて微量ではあるが動物の肝臓中にビタミンB12の成分として含まれる。[鳥居泰男]
-
溶解性
水および有機溶媒に不溶。希塩酸, 希硫酸, 希硝酸に水素を発生して溶ける。希塩酸及び希硫酸には徐々に溶け、硝酸には溶けやすい。
-
主な性質
- 単体コバルトは灰白色の金属で、酸化物の色は黄色か黒
- コバルトは化学的性質にはニッケル及び鉄に近い
- コバルトは通常は空気や水の影響を受けない(硫酸、塩酸、硝酸等に激しく反応し、アルカリには溶けない)
- 硬度、引張強度、切削性、熱的性質もニッケルや鉄に似ている
- コバルトは強磁性合金である
- コバルトにニッケル、クロム及びモリブデンを含む合金は、高温強度が強い
- コバルトにクロム、タングステン、鉄を含む合金は耐クリープ性、耐摩耗性、耐食性が高い(切削道具に広く用いられる)
- コバルト?クロム基合金は人の体液と反応しない(医療に用いられる)
-
用途
触媒、合金材料。
-
用途
合金材料、磁性材料、触媒。
-
用途
合金結合材、耐熱材料、磁性材料、触媒。
-
製法
金属コバルトの抽出法は原鉱の性質によって多様であるが、一般には、まず精選した鉱石を電気炉で融解処理して金属成分を鈹(かわ)、砒鈹または合金の形で濃縮し、濃硫酸などに溶かす。適当な化学処理で共存する他の金属成分を分離して水酸化コバルトとして沈殿させ、約800℃に加熱して酸化コバルトCo2O3とする。これを約1000℃で木炭で還元すると粒状コバルトが得られる。また、約700℃で水素還元すると粉状となる。化学的工程のかわりに溶液を鉛陽極で電解してコバルトを回収する方法も行われている。いずれによっても純度は99%以上である。より高純度のコバルトは、硫酸コバルト(Ⅲ)溶液の電解還元、コバルトカルボニルの熱分解などによって得られる。ほかにクロロペンタアンミンコバルト(Ⅲ)塩化物[CoCl(NH3)5]Cl2に変え、熱分解する方法も用いられる。
-
用途
純金属は、たとえばフィッシャー‐トロプシュ合成用触媒などに用いられるが、むしろ各種合金の成分としての用途のほうが重要である。高速度鋼、焼結炭化物合金などの切削工具用の超硬質材料、KS鋼、モリブデン‐コバルト鋼などの磁石材料の製造に用いられる。また、高温での耐酸化性、耐食性、耐摩耗性に優れた超耐熱合金(超合金)が種々開発されているが、コバルト25~65%を含む非鉄合金もその一つである。原子炉中で金属コバルトに中性子を照射すると、半減期5.2年の放射性同位体コバルト60を人工的につくることができる。これが放出するγ(ガンマ)線は強力でかつ安価でもあるので、ラジウムにかわってγ線源として放射線化学の研究のほか、理化学、工学、生物学など広い分野で利用されている。γ線を照射して遺伝学などの研究を行うものを、とくにγ農場といっている。また、人体の深部まで浸透するので腫瘍(しゅよう)の治療に用いられる。
人体に約2ミリグラム含まれ、主としてビタミンB12の構成成分として存在する。ビタミンB12は抗貧血性ビタミンで、コバルトの欠乏症はビタミンB12欠乏症の悪性貧血である。また、過剰症には悪心(おしん)や発疹(はっしん)、聴覚障害などがある。
-
主な用途
- 超硬合金工具(切削工具、耐磨工具)
- 特殊鋼部品(高速度鋼、一般工具、工作機械部品、航空機エンジン部品、ガスタービン部品)
- 磁性材料部品(永久磁石、テレビ、音響部材、磁気ディスク)
- 化成品(触媒、磁気テープ)
- その他(顔料、ガラスの消光剤、ホウロウの下塗)
-
説明
Cobalt was discovered by George Brandt in 1737. Cobalt exists
in valence states from 0 to 5, with the most stable (+2 and +3)
being the most common. Although there is only one stable
isotope of cobalt, there are a number of unstable isotopes. Two
of these, cobalt-60 and cobalt-57, are in use commercially.
Cobalt-60 is used for cancer treatment and food irradiation.
Cobalt-57 has research applications.
-
化学的特性
Cobalt is a silver-gray to black, hard, brittle, magnetic metal. It is relatively rare; the important mineral sources are the arsenides, sulfides, and oxidized forms. It is generally obtained as a by-product of other metals, particularly copper. The fume and dust of cobalt metal is odorless and black. The appearance and odor of cobalt compounds and their dusts and fumes vary with the compound. Cobalt metal in powdered form is incompatible with fused ammonium nitrate, hydrozinium nitrate, and strong oxidizing agents and should be avoided. It ignites on contact with bromide pentafl uoride. Powdered cobalt ignites spontaneously in air. Exposure to cobalt metal fume and dust can occur through inhalation, ingestion, and eye or skin contact.
-
物理的性質
Cobalt is a bluish steel-gray metal that can be polished to a bright shine. It is brittle andis not malleable unless alloyed with other metals. It is magnetic, and when alloyed with aluminum and nickel, it is called alnico metal, which acts as a super-magnet with many uses inindustry. Chemically and physically, cobalt acts much as do its two partners, iron (Fe) andnickel (Ni), located on each side of it in period 4 on the periodic table. In particular, iron,cobalt, and nickelare unique in that they possess natural magnetic properties. Cobalt’s meltingpoint is 1,495°C, its boiling point is 2,927°C, and its density is 8.86 g/cm3.
-
同位体
There are 33 isotopes of cobalt, ranging from Co-48 to Co-75, with half-livesranging from a few nanoseconds to 5.272 years for cobalt-60. Cobalt-59 is the onlystable isotope that constitutes almost all (roughly 100%) of the element’s natural presence on Earth. All the other isotopes are radioactive and are created artificially in nuclearreactors or nuclear explosions.
-
名前の由来
Cobalt was given the name kobolds (or kolalds, or kololos) by German
miners. It means “goblin” (see “History” for more on this story).
-
天然物の起源
Cobalt is the 32nd most abundant element on Earth even though it makes up only 0.003%of the Earth’s crust. It is not found in the free metallic state, despite being widely distributedin igneous rocks as minerals. Its two most common mineral ores are cobaltite (CoAsS) anderythrite [Co3(AsO4)2]. These ores are placed in blast furnaces to produce cobalt arsenide(Co2As), which is then treated with sulfuric acid to remove the arsenic. Finally, the productcobalt tetraoxide (Co3O4) is reduced by heat with carbon (Co3O4 + C → 3Co + 2CO2), resulting in cobalt metal.Cobalt is also found in seawater, meteorites, and other ores such as linnaeite, chloanthite,and smaltite, and traces are found mixed with the ores of silver, copper, nickel, zinc, andmanganese. Cobalt ores are found in Canada and parts of Africa, but most of the cobalt usedin the United States is recovered as a by-product of the mining, smelting, and refining of theores of iron, nickel, lead, copper, and zinc.
-
特性
Cobalt has the highest Curie point of any metal or alloy of cobalt. The Curie point is thetemperature at which an element will lose its magnetism before it reaches its melting point.Cobalt’s Curie point is 1,121°C, and its melting point is 1,495°C. About 25% of all cobaltmined in the world is used as an alloy with other metals. The most important is the alloyalnico, which consists of nickel, aluminum, and cobalt. Alnico is used to make powerful permanent magnets with many uses, such as CT, PET, and MRI medical instruments. It is alsoused for electroplating metals to give a fine surface that resists oxidation.
-
使用
For alloys; manufacture of cobalt salts; in nuclear technology. Since 60Co can be encapsulated compactly, it has replaced radium in experimental medicine and cancer research. Cobalt is also used in the cobalt bomb, a hydrogen bomb surrounded by a cobalt metal shell. When the nuclear explosion occurs 60Co is formed from 59Co by neutron capture. Considered a "dirty bomb" because of long half-life and intense b- and g radiation. Max permissible concentration of 60Co in air: 10-7mCi/cc, Natl. Bur. Stand. Handb. 69, 31 (1959).
-
調製方法
World sources of the metal and the oxide are chiefly from
Zaire, Belgium–Luxembourg, Norway, and Finland, in that
order, with Zaire furnishing 58% of the world’s supply.
Practically all cobalt produced is a by- or coproduct of
other metals, chiefly copper; accordingly, a description of the
mining process is omitted. The processes used in extracting
cobalt from its ores vary according to the type of ore and
locations of the ore deposit.
Arsenical ores are concentrated by hand sorting, gravity
separation, or froth flotation, and are smelted in a blast
furnace with coke and limestone to a speiss (an impure
mixture of iron, cobalt, and nickel arsenides). The speiss is
ground, roasted with salt, and leached with water. Insoluble
chlorides remaining after the leaching process are
ground with sulfuric acid, washed, and filtered, and the
washings are added to the liquid from the leaching step.
The combined solution is oxidized and then neutralized
with lime.
Basic ferric arsenate precipitates and is removed, leaving
a solution-containing cobalt and nickel. The addition of
successive portions of sodium hydroxide and sodium hypochlorite
precipitates cobalt as the hydroxide, which is
initially pure but finally admixes with nickel hydroxide.
The cobalt precipitate is dried, ground, and formed into
pellets, which are reduced by heating with charcoal to
cobalt metal.
-
定義
ChEBI: A cobalt group element atom that has atomic number 27.
-
反応性
Cobalt absorbs very little hydrogen even at high temperatures and nitrogen is practically
insoluble up to 1200°C. Finely divided cobalt is pyrophoric in air, but the massive metal
is scarcely attacked below 300°C. The oxide scale on cobalt heated in air or oxygen up to
900° consists of an outside layer of CO3O4 and a layer of CoO next to the metal ; above
900°, Co3O4 decomposes and the scale consists of CoO only. Cobalt reacts with many
non-metals when heated, e.g. the halogens, boron, sulphur, phosphorus, arsenic and
antimony, the reactions often proceeding with incandescence. Fluorine forms CoF3, while
the other halogens give the cobalt(II) halide.
-
世界保健機関(WHO)
WHO Comment(non-radioactive forms): The World Health Organization has no information further to the
above regarding preparations containing cobalt or to indicate that they are still
commercially manufactured.
-
空気と水の反応
Burns brilliantly when exposed to air [Mellor 14:453(1946-1947)]. Insoluble in water.
-
反応プロフィール
Pyrophoric Cobalt is a reducing agent. Decomposes acetylene in the cold as the metal becomes incandescent [Mellor 14:513(1946-1947]. Incompatible with oxidizing agents such as ammonium nitrate, bromine pentafluoride, and nitryl fluoride.
-
危険性
Cobalt is found in most natural foods. Although a necessary trace element, it is toxic to humans if ingested in large amounts. The human body does excrete in urine excessive amounts of cobalt compounds such as found in vitamin B12.
Cobaltous chromate (CoCrO4) is brownish-yellow to grayish-black (the color depends on its purity) is a dangerous carcinogen (causes cancer).
Some years ago, a cobalt additive was used by some beer makers to maintain a foam head on their beer. Those who imbibed excessively developed what is known as beer drinkers syndrome, which caused some deaths from enlarged and flabby hearts.
-
健康ハザード
Cobalt is an essential element. Its deficiencycan result in pernicious anemia. It is present invitamin B12. Excessive intake of this elementmay result in polycythemia or overproductionof erythrocytes and heart lesions. Exposure toits dusts can produce cough and respiratoryirritation. Chronic inhalation of its dusts orfumes can decrease pulmonary functions andmay cause diffuse nodular fibrosis and otherpulmonary diseases. Skin contact may inducedermal hypersensitivity reactions, producingan allergy-type dermatitis.
Co(II) ion is reported to be genotoxicin vitro and in vivo and carcinogenic inrodents (De Boeck et al. 2003) Occupationalexposure to hard metal (cemented carbide)dust is linked to an increased risk of lungcancer.
-
火災危険
Literature sources indicate that the dust of Cobalt is flammable.
-
工業用途
Cobalt (symbol Co) is a lustrous, silvery-bluemetallic chemical element, resembling nickelbut with a bluish tinge instead of the yellow ofnickel. It is rarer and costlier than nickel andits price has varied widely in recent years.Although allied to nickel, it has distinctive differences.It is more active chemically thannickel. It is dissolved by dilute H2SO4, HNO3,or HCl acids, and is attacked slowly by alkalis.The oxidation rate of pure cobalt is 25 timesthat of nickel. Its power of whitening copperalloys is inferior to that of nickel, but smallamounts in Ni–Cu alloys will neutralize theyellowish tinge of the nickel and make themwhiter. The metal is diamagnetic like nickel, buthas three times the maximum permeability.Like tungsten, it imparts red-hardness to toolsteels. It also hardens alloys to a greater extentthan nickel, especially in the presence of carbon,and can form more chemical compoundsin alloys than nickel.
Its chemical properties resemble, in part,those of both nickel and iron. Cobalt is themetal with the highest Curie temperature(1121°C) and the lowest allotropic transformationtemperature (399°C). Below 421°C, cobaltis close-packed hexagonal; above, it is facecenteredcubic.
-
生物活性
Cobalt is a vital trace element in animal nutrition. Ruminants grazing upon cobaltdeficient pastures exhibit retarded growth, loss of appetite and anaemia ; rapid recovery from these symptoms occurs upon feeding the animals with a cobalt-supplemented diet. Cobalt salts are not therefore considered to be particularly toxic to animals, but to man they can in sufficiently large doses irritate the gastro-intestinal tract and cause nausea, vomiting and diarrhoea. Small amounts of cobalt, however, are invaluable in the treatment of pernicious anaemia. The discovery in 1926 of the antipernicious anaemia factor in liver led to the discovery in 1948 of vitamin B12, which was very soon after shown to contain cobalt.
-
安全性プロファイル
Confirmed carcinogen
with experimental neoplastigenic and
tumorigenic data. Poison by intravenous,
intratracheal, and intraperitoneal routes.
Moderately toxic by ingestion. Inhalation of
the dust may cause pulmonary damage. The
powder may cause dermatitis. Ingestion of
soluble salts produces nausea and vomiting
by local irritation. Powdered cobalt igmtes
spontaneously in air. Flammable when
exposed to heat or flame. Explosive reaction
with hydrazinium nitrate, ammonium nitrate
+ heat, and 1,3,4,7-tetramethylisoindole (at
39OOC). Ignites on contact with bromine
pentafluoride. Incandescent reaction with
acetylene or nitryl fluoride. See also
COBALT COMPOUNDS.
-
職業ばく露
Possible risk of forming tumors, Suspected reprotoxic hazard. Nickel-aluminumcobalt alloys are used for permanent magnets. Alloys with nickel, aluminum, copper, beryllium, chromium, and molybdenum are used in the electrical, automobile, and aircraft industries. Cobalt is added to tool steels to improve their cutting qualities and is used as a binder in the manufacture of tungsten carbide tools. Various cobalt compounds are used as pigments in enamels, glazes, and paints; as catalysts in afterburners; and in the glass, pottery, photographic, electroplating industries. Radioactive cobalt (60Co) is used in the treatment of cancer. Cobalt has been added to beer to promote formation of foam but cobalt acts with alcohol to produce severe cardiac effects at concentrations as low as 1.2-1.5 mg/L of beer. Cobalt is part of the vitamin B12 molecule and as such is an essential nutrient. The requirement of humans for cobalt in the form of vitamin B12 is about 0.13 μg/day.
-
導入
化合物としてガラスなどを独特の青色にする性質は古代エジプトから知られ、今でも陶磁器の着色剤として使われている。鮮やかな青色の空を表現する時、「コバルトブルー」という表現が用いられる。コバルトは強磁性体、白色の金属で鉄より酸化されにくく、酸やアルカリにも強い。
-
特性
・強磁性
・酸、アルカリに強
い
・腐食に強い
-
用途
自動車電動化に欠かせない LIB 正極材や特殊鋼(スーパーアロイ)に使用
コバルトは強磁性体、白色の金属で鉄より酸化されにくく、酸やアルカリにも強い。
コバルトは国内では携帯電話、ノートパソコン、EV等に使用されるLIB正極材の用途
が最も多い。そのほかの用途は、超硬合金のバインダー、高速度鋼や耐熱鋼等の
特殊鋼添加剤、HDD 等の磁性材、家庭電化製品・音響機器等に使用されるアルニコ
磁石(Al-Ni-Co)やサマリウム・コバルト磁石等の永久磁石、石油精製時の脱硫触媒
等である。また耐熱性に優れることから欧米等においては約半数がジェットエンジン
用の超合金向けとなっている。
-
発がん性
In mammalian cells in vitro cobalt compounds
have caused DNA strand breaks, sister
chromatid exchanges, and aneuploidy, but
not chromosomal aberrations.Cobalt salts
are generally nonmutagenic in prokaryotic
assays.
-
準備
コバルトの製法には、銅・コバルトの酸化鉱、硫化鉱からの精錬、ニッケル製錬時の副産物からの精錬などがある。銅・コバルトの酸化鉱は、精鉱を硫酸液に浸し銅・コバルトを抽出し、銅を電解採取し不純物を除去した後に、金属コバルトを電解採取する。硫化物からの製錬は、酸化焙焼した後、温湯でコバルトを抽出し、石灰を加え水酸化コバルトを沈殿させる。また、ラテライト鉱からのニッケル製錬で発生する Ni-Co 混合硫化精鉱(ミックスサルファイド)について、溶媒抽出法により Ni-Co 塩酸溶液を作った後、ニッケルとコバルトを分離、塩化ニッケルと塩化コバルトにし、電解採取により電気ニッケルと電気コバルトを製造する。
-
環境運命予測
Cobalt most often depresses the activity of enzymes, including
catalase, amino levulinic acid synthetase, and P-450, enzymes
involved in cellular respiration. The Krebs citric acid cycle can
be blocked by cobalt resulting in the inhibition of cellular
energy production. Cobalt can replace zinc in a number of zincrequired
enzymes such as alcohol dehydrogenase. Cobalt can
also enhance the kinetics of some enzymes, such as heme
oxidase in the liver. Cobalt interferes with and depresses iodine
metabolism, resulting in reduced thyroid activity. Reduced
thyroid activity can lead to goiter.
-
貯蔵
Cobalt metal dust (powdered metal) should be stored in a cool, dry, well-ventilated area in
tightly sealed containers that are labeled in accordance with OSHA standards. Containers
of cobalt metal dust should be protected from physical damage and ignition sources, and
should be stored separately from strong oxidizers.
-
輸送方法
UN3189 Metal powder, self-heating, n.o.s., Hazard Class: 4.2; Labels: 4.2-Spontaneously combustible material
-
廃棄物の処理
Cobalt metal may be recovered from scrap and cobalt compounds from spent catalysts as alternatives to disposal.