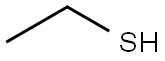
-
外観
無色~ほとんど無色, 澄明の液体
-
性質
エタンチオールの色は無色で、非常に強い刺激臭を持ち、かなり遠くからでも認識できるほどの悪臭を呈します。この化合物は、世界で最も臭い化合物としてギネス世界記録にも認定されています。
エタンチオールは、硫酸エチルカリウムに硫化水素カリウムを反応させることでエタンチオールを得ることが可能です。また、その他のエタンチオールは、ハロエタンに硫化水素カリウムを反応させることでも、エタンチオールを製造することができます。
-
溶解性
水に微溶 (0.68g/100ml 20℃), アルコール, エーテルに可溶。水及びエタノールに溶ける。
-
解説
ethyl thioalcohol.C2H6S(62.14).C2H5SH.エチルチオアルコール,エチルメルカプタンともいう.ハロエタンまたは硫酸エチルカリウムに硫化水素カリウム(KSH)を作用させて合成する.エタンチオールは,悪臭のある無色の揮発性液体.融点-144 ℃,沸点35 ℃.d425"0.83147.nD20"1.431.エタノール,エーテル,アルカリ水溶液に可溶.弱酸性で,金属チオラートを生じる.空気または希硫酸によって酸化されジエチルジスルフィドを,また濃硝酸によりエタンスルホン酸を与える.アルデヒド,ケトンなどと酸の存在下で反応し,チオアセタール,チオケタールを生じる.都市ガスに臭いをつけるため,添加剤として用いられる.森北出版「化学辞典(第2版)
-
用途
燃料用ガス,特にLPGの付臭剤として使用農薬,医薬,ゴム薬原料の合成及びエチルチオグループの製造に応用
-
用途
有機合成原料。
-
危険性
エタンチオールは極めて引火性が高く、また体内に吸入してしまうと人体に有害です。この化合物は消防法に定める「第4類危険物特殊引火物」に該当しており、取り扱う際にはドラフト内で取り扱うことや、近くに高温のものを置かないなどといったことを徹底する必要があります。また、皮膚や目への刺激を回避するために、白衣や保護メガネを着用することが重要です。
-
説明
Ethane thiol, commonly known as ethyl mercaptan, is a colorless gas or clear liquid with a distinct odor. It is an organosulfur compound with the formula CH3CH2SH. Abbreviated EtSH, it consists of an ethyl group (Et), CH3CH2, attached to a thiol group, SH. Its structure parallels that of ethanol, but with S instead of O. The odor of EtSH is infamous. Ethanethiol is more volatile than ethanol due to a diminished ability to engage in hydrogen bonding. Ethanethiol is toxic. It occurs naturally as a minor component of petroleum, and may be added to other wise odorless gaseous products such as liquefied petroleum gas (LPG) to help warn of gas leaks. At these concentrations, ethanethiol is not harmful.
-
物理的性質
Colorless liquid with a strong, disagreeable, skunk-like or rotten egg odor. Extremely flammable
liquid or gas. An experimentally determined odor threshold concentration of 1 ppbv was reported
by Leonardos et al. (1969). Katz and Talbert (1930) reported experimental detection odor
threshold concentrations in the range 0.66–7.6 μg/m3 (0.26 to 3.0 ppbv).
-
使用
Ethanethiol is used as an intermediate inthe manufacture of insecticides, plastics, andantioxidants; and as an additive to natural gasto give odor. It occurs in illuminating gas andin petroleum distillates.
-
調製方法
Ethyl mercaptan is prepared by distilling ethyl potassium sulfate with potassium hydrogen sulfide. Additional mercaptans can be prepared in a similar manner with the corresponding proper ingredients.
-
製造方法
Ethanethiol is prepared by the reaction of ethylene with hydrogen sulfide over a catalyst. The various producers utilize different catalysts in this process. It is also be prepared commercially by the reaction of ethanol with hydrogen sulfide gas over an acidic solid catalyst, such as alumina.
Ethanethiol was originally reported by Zeiss in 1834 . Zeise treated calcium ethyl sulfate with a suspension of barium sulfide saturated with hydrogen sulfide. He is credited with naming the C2H5S- group as mercaptum.
Ethanethiol can also be prepared by a halide displacement reaction, where ethyl halide is reacted with aqueous sodium bisulfide. This conversion was demonstrated as early as 1840 by Henri Victor Regnault.
-
定義
ChEBI: Ethanethiol is an alkanethiol that is ethane substituted by a thiol group at position 1. It is added to odorless gaseous products such as liquefied petroleum gas (LPG) to provide a garlic scent which helps warn of gas leaks. It has a role as a rodenticide.
-
反応性
Ethane thiol is a valued reagent in organic synthesis. In the presence of sodium hydroxide, it gives the powerful nucleophile SEt-. The salt can be generated quantitatively by reaction with sodium hydride.
Ethane thiol can be oxidized to ethyl sulfonic acid, using bleach and related strong aqueous oxidants. Weaker oxidants, such as ferric oxide give the disulfide, diethyl disulfide :
2 EtSH + H2O2 → EtS-SEt + 2 H2O
Like other thiols, it behaves comparably to hydrogen sulfide. For example, it binds, concomitant with deprotonation to "soft" transition metal cations, such as Hg2+, Cu+, and Ni2+ to give polymeric thiolato complexes, Hg(SEt)2, CuSEt, and Ni(SEt)2, respectively.
-
一般的な説明
A clear colorless low-boiling liquid (boiling point 97°F) with an overpowering, garlic-like/skunk-like odor. Flash point -55°F. Less dense than water and very slightly soluble in water. Vapors are heavier than air. Vapors may irritate nose and throat. May be toxic if swallowed, by inhalation or by contact. Added to natural gas as an odorant. Used as a stabilizer for adhesives.
-
空気と水の反応
Highly flammable. A very dangerous fire hazard. Very slightly soluble in water.
-
反応プロフィール
Ethanethiol reacts violently with calcium hypochlorite, May react vigorously with other oxidizing reagents. On contact with strong acids or when heated to decomposition Ethanethiol emits highly toxic fumes of sulfur oxides [Sax, 9th ed., 1996, p. 1575].
-
危険性
Toxic by ingestion and inhalation.
Flammable, dangerous fire risk.
-
健康ハザード
The inhalation toxicity of ethanethiol islower than that of methanethiol. Intraperitoneal administration in rats at sublethaldoses caused deep sedation, followed bylethargy, restlessness, lack of muscular coordination, and skeletal muscle paralysis.Higher doses produced cyanosis, kidney andliver damage, respiratory depression, coma,and death. The intraperitoneal LD50 value inrats was 450 mg/kg (ACGIH 1986).Ethanethiol is metabolized to inorganicsulfate and ethyl methyl sulfone, and excreted. The oral LD50 value in rats is 680 mg/kg.In humans, repeated exposures to itsvapors at about 5 ppm concentration canproduce irritation of the nose and throat,headache, fatigue, and nausea.
-
使用用途
1. 都市ガス
エタンチオールは都市ガスに添加することで、都市ガスに臭いをつけることができます。上記のようにエタンチオールは悪臭をもち、その臭いはタマネギやニラの臭いに近い強い刺激臭です。
このエタンチオールの性質を利用して、ガスに添加することで、ガス漏れした時にいち早く気づくことができます。エタンチオールがガスの付臭剤として使われるようになった理由は、アメリカの石油会社従業員が偶然にガス漏れをしている部分にヒメコンドルが集まっていることを発見したからです。
このヒメコンドルは、のちにエタンチオールの臭いに集まっていることが判明しました。それ以降、ガス中のエタンチオールの濃度を高くして、人間にも臭いが感知できるようにすることで、ガス漏れの検知にエタンチオールが使われるようになりました。
2. 有機合成
エタンチオールは、有機合成における試薬としても使用されます。エタンチオールは非常に反応性の高いチオール基を持っているため、スルフィド構造をもつ化合物やチオエステル構造を持っている化合物を合成する際に利用できます。ただし、高すぎる反応性を持つことによって副反応が起きる可能性もあるので、保護基をうまく導入するなどの工夫を行うことが必要です。
しかし、この化合物は沸点が36℃と低いことや、強い刺激臭を持っていることから、取り扱いには注意が必要です。
-
類似物
1,1-エタンジチオールの性質
1,1-エタンジチオールは、エタンチオールのチオール基が結合する炭素に、さらにチオール基が1つ結合した化合物です。この物質はエタンチオールに類似した構造を持ちますが、性質も類似しており非常に強い悪臭を持ちます。
この化合物を含む有名が、果物の王と呼ばれているドリアンです。この物質が含まれていることを同定した方法としては、まずドリアンの果肉にジクロロメタンを分散させて有機物を溶解し、硫酸ナトリウムで脱水を行います。この抽出液を40℃で蒸留を行い、揮発性の匂いを持つ化合物を分離します。これらの化合物をガスクロマトグラフィーで保持時間および分子量を分析することによって、化合物が決定されました。
参考文献
https://pubchem.ncbi.nlm.nih.gov/compound/6343
-
化学反応性
Reactivity with Water No reaction; Reactivity with Common Materials: No reaction; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.
-
安全性プロファイル
Moderately toxic by
ingestion, inhalation, and intraperitoneal
routes. A skin and eye irritant. Inhalation
causes central nervous system effects in
humans. A very dangerous fire hazard when
exposed to heat or flame; can react
vigorously with oxidizing materials. A
moderate explosion hazard when exposed to
spark or flame. Violent reaction with
Ca(OCl)2. Will react with water or steam to
produce toxic and flammable vapors. To
fight fire, use CO2, dry chemical. When
heated to decomposition or on contact with
acid or acid fumes it emits hghly toxic
fumes of SOx. See also MERCAPTANS.
-
職業ばく露
This material is used as a warning
odorant for liquefied petroleum gases. It is used as an intermediate in the manufacture of many pesticides and other
organic chemicals
-
環境運命予測
Biological. Ethyl mercaptan did not degrade in anaerobic sediments and sludges nor in
anaerobic freshwater conditions (van Leerdam et al., 2006).
Photolytic. A second-order rate constant of 1.21 x 10-12 cm3/molecule?sec was reported for the
reaction of ethyl mercaptan and NO3 radicals in the atmosphere at 297 K (Atkinson, 1991).
Chemical/Physical. In the presence of nitric oxide, ethyl mercaptan reacted with OH radicals
forming ethyl thionitrite. The rate constant for this reaction is 2.7 x 10-11 at 20 °C (MacLeod et al.,
1984).
-
輸送方法
UN2363 Ethyl mercaptan, Hazard Class: 3;
Labels: 3-Flammable liquid
-
純化方法
Dissolve the thiol in aqueous 20% NaOH, extract it with a small amount of *benzene and then steam distil until clear. After cooling, the alkaline solution is acidified slightly with 15% H2SO4 and the thiol is distilled off, dried with CaSO4, CaCl2 or 4A molecular sieves, and fractionally distilled under nitrogen [Ellis & Reid J Am Chem Soc 54 1674 1932]. It has a foul odour. [Beilstein 1 IV 1390.]
-
不和合性
May form explosive mixture with air.
Slowly forms peroxides. This material is a weak acid.
Reacts with oxidizers, causing fire and explosion hazard.
Reacts with strong acids evolving toxic and flammable
hydrogen sulfide. May accumulate static electrical charges,
and may cause ignition of its vapors. Attacks some forms
of plastics, coatings and rubber. Aldehydes are frequently
involved in self-condensation or polymerization reactions.
These reactions are exothermic; they are often catalyzed by
acid. Aldehydes are readily oxidized to give carboxylic
acids. Flammable and/or toxic gases are generated by the
combination of aldehydes with azo, diazo compounds,
dithiocarbamates, nitrides, and strong reducing agents.
Aldehydes can react with air to give first peroxo acids, and
ultimately carboxylic acids. These autoxidation reactions
are activated by light, catalyzed by salts of transition
metals, and are autocatalytic (catalyzed by the products of
the reaction). The addition of stabilizers (antioxidants) to
shipments of aldehydes retards autoxidation
-
廃棄物の処理
Incineration (1093℃�) followed by scrubbing with a caustic solution