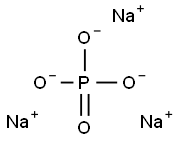-
種類
リン酸ナトリウムには、無水和物 (Na3PO4) の他に一水和物 (Na3PO4・H2O) 、六水和物 (Na3PO4・6H2O) 、七水和物 (Na3PO4・7H2O) 、十水和物 (Na3PO4・10H2O) 、十二水和物 (Na3PO4・12H2O) など多くの水和物があります。このうち市販されているものは、主に無水和物 (無水) 、六水和物 (六水塩) 、十二水和物 (十二水塩) です。
リン酸ナトリウム (無水) は、医薬部外品原料規格、食品添加物、1級、工業用などの規格で販売されている物質です。
リン酸ナトリウム (六水塩) は、主に工業用の規格で販売されている物質です。
リン酸ナトリウム (十二水塩) は、医薬部外品原料規格、食品添加物、試薬特級、1級、工業用などの規格で販売されています。
-
定義
本品は、リン酸(*)の三ナトリウム塩であり、次の化学式で表される。
参照表示名称:リン酸
-
性質
リン酸ナトリウムは水によく溶けます。25℃の水100グラムに対するリン酸ナトリウムの溶解度は14.5です。水溶液は強アルカリ性 (pH11.5〜12.5) で、酸と激しく反応します。
リン酸ナトリウムの密度は2.5グラム/立方センチメートル、融点は1340℃です。加熱により分解し、リン酸化物などの有毒で腐食性のヒュームを生じます。エタノールに不溶です。
-
解説
リン酸ナトリウム,無水和物は,白色ないし無色の六方晶系結晶.密度2.735 g cm-3.融点1340 ℃.溶解度14.5 g/100 g 水(25 ℃).水溶液は加水分解により強アルカリ性を呈する.エタノールに不溶.十二水和物は,白色の六方晶系結晶.密度1.62 g cm-3.~75 ℃ で分解する.100 ℃ に加熱すると一水和物になる.アルカリ性洗浄剤,皮なめし剤,染色助剤,染料分散剤,果実剥皮剤,砂糖精製,清缶剤,硬水処理剤などとして用いられる.
-
用途
清缶剤,食品添加物(ベーキングパウダー),医薬部外品原料,染色用媒染剤
-
化粧品の成分用途
pH調整剤、キレート剤、緩衝剤、消臭剤
-
製造
リン酸ナトリウム,略称TSP.リン酸三ナトリウム(trisodium phosphate),第三リン酸ナトリウム,第三リン酸ソーダともいう.このほか,リン酸水素二ナトリウム(disodium hydrogenphosphate)(略称DSP)Na2HPO4,およびリン酸二水素ナトリウム(sodium dihydrogenphosphate)(略称MSP)NaH2PO4がある.リン酸水素二ナトリウムNa2HPO4を単にリン酸ナトリウムとよぶこともある.三ナトリウム塩は,リン酸に過剰の水酸化ナトリウムを加えるか,リン酸水素二ナトリウム水溶液と水酸化ナトリウム水溶液を1:1モル比で反応させるなどの方法により得られる.常温では十二水和物が得られるが,このほか,無水和物,0.5,一,六,八,十水和物が知られている.十二水和物は100 ℃ で一水和物に,200 ℃ で無水和物になる.
-
合成法
リン酸と水酸化ナトリウムの反応
H3PO4 + 3NaOH → Na3PO4 + 3H2O
この方法で生成したリン酸ナトリウムを蒸発濃縮すると、常温で十二水和物が得られます。十二水和物は100℃に加熱すると一水和物になり,さらに200℃まで加熱すると無水和物になります。
塩化水素との反応
Na3PO4 + 3HCl → 3NaCl + H3PO4
過塩素酸との反応
Na3PO4 + 3HClO4 → 3NaClO4 + H3PO4
酸化ナトリウムと十酸化四リンの生成反応
4Na3PO4 → 6Na2O + P4O10
-
効能
下剤
-
説明
Trisodium phosphate (TSP) is an inorganic salt used as industrial detergents, metal treatment and in toilet floor cleaners. TSP is pure cleaning power. Used as a water softener; for the treatment of boiler water; as a paint remover; in photographic developers; for tanning leather; for manufacturing paper; for clarifying sugar.
Also, it is a common laboratory reagent. Use to dissolve dirt, grease, and mildew from siding, decks, masonry, boats, campers. To prepare surfaces before painting or staining wash with TSP. Trisodium phosphate is an approved food additive in the U.S., European Union and other countries of the world. It may be added to foods and beverages or smoothies and green drinks. The primary function of trisodium phosphate is acidity regulation. It is commonly present in dry, extruded cereals. Together with other phosphates, it modifies cereal color, aids the cereal's flow through the extruder and provides phosphorus fortification. It is also commonly present in cheese sauces as an emulsifier. Trisodium phosphate is a strong chemical and can cause severe eye damage and can burn unprotected skin. Poisoning occurs if you swallow, breathe in, or spill large amounts of this substance on your skin.
-
化学的特性
Trisodium phosphate (anhydrous) is a white, granular or crystalline solid, highly soluble in water and produces a strong alkaline solution. On exposure to heat, trisodium phosphate decomposes and produces toxic and corrosive fumes including phosphorous oxides.
The major use for trisodium phosphate is as a cleaning agent, food additive, stain remover, and degreaser. Trisodium phosphate of commercial grade is often partially hydrated and ranges from anhydrous trisodium phosphate, Na3PO4, to the dodecahydrate, Na3PO4 · 12H2O. Most often found in white powder form, it is also called trisodium orthophosphate or just plain sodium phosphate. Trisodium phosphate reacts violently with water and acids to liberate heat. Trisodium phosphate is corrosive and in the presence of water attacks many metals.
Trisodium phosphate is an approved flux for use in hard soldering joints in medical grade copper plumbing. The flux is applied as a concentrated water solution and dissolves copper oxides at the temperature used in copper brazing. Residues are fully water soluble and can be rinsed out of plumbing before it is put in service. Also, trisodium phosphate is still in vast use for the cleaning, degreasing, and deglossing of walls prior to painting. In fact, application of trisodium phosphate breaks the gloss of oil-based paints and opens the pores of latex-based paint providing a surface better suited for the adhesion of the subsequent layer of paint.
-
物理的性質
The dodecahydrate is a white or colorless hexagonal crystal; density 1.62 g/cm3; melts around 75°C on rapid heating; partially loses water of crystallization at 100°C; retains the last water molecule even at moderate ignition; soluble in water, about 28 g/100 mL at 20°C; the solution is strongly alkaline; the pH of a 0.1M solution 11.5; insoluble in alcohol.
-
使用
Trisodium phosphate (TSP) is a cleaning agent, lubricant, food additive, stain remover and degreaser. It is an alkaline cleaning agent that has been used as a household cleaner for many years, but ecological problems have largely ended that practice, at least in the western world. Substitutes are not as effective, but the raw chemical can be bought in bulk to add to other detergents. It works by disrupting the bacterial cell membrane and causing the contents to leak out, though the exact mechanism is not fully elucidated (Oyarzabal, 2005). Trisodium phosphate solutions are approved for treatment of beef carcasses in the US Code of Federal Regulations (21 CFR 182.1778; FDA 2003).
By the end of the 20th century, many products that formerly contained TSP were manufactured with TSP substitutes, which consist mainly of sodium carbonate along with various admixtures of nonionic surfactants and a limited percentage of sodium phosphates. TSP is commonly used after cleaning with mineral spirits in order to remove hydrocarbon residues. TSP may be used with household chlorine bleach in the same solution without hazardous reactions. This mixture is particularly good for removing mildew, but is ineffective at permanently removing mold.
-
製造方法
Trisodium phosphate may be prepared in two steps, first by adding a little excess of sodium carbonate to phosphoric acid and then boiling the solution to expel carbon dioxide. Sodium hydroxide is then added to the solution:
Na2CO3+ H3PO4→Na2HPO4+ CO2+ H2O
Na2HPO4+ NaOH →Na3PO4+ H2O
Alternatively, trisodium phosphate may be prepared by complete neutralization of phosphoric acid with sodium hydroxide, followed by evaporation and crystallization:
H3PO4+ 3NaOH →Na3PO4+ 3H2O
-
一般的な説明
Sodium phosphate is a colorless to white crystalline powder or granules. It is prepared by neutralization of phosphoric acid under controlled conditions with sodium hydroxide or sodium carbonate .
-
危険性
Toxic by ingestion, irritant to tissue.
-
使用用途
1. 食品分野
リン酸ナトリウムは、食品添加物としてハムやソーセージなどの食肉の結着剤として使われる物質です。また、かんすいの添加剤やpH調整剤としても使用されています。
2. 工業分野
写真用現像液や製紙薬剤、皮なめし剤、染色助剤などに広く使われています。
その他、医薬品の添加物や医薬部外品の原料、リン酸系の洗剤、植物のウイルス病対策のための消毒液などに使用されています。
-
工業用途
Different salts of phosphoric and polyphosphoric acids are used in flotation. From this
fairly large family of reagents, sodium phosphate is the preferred species.
Trisodium phosphate is a white, crystalline substance highly soluble
in water. Neutralizing phosphoric acid with soda ash produces trisodium phosphate.
Mono- and disodium
phosphates are rarely used.
-
作用機序
TSP is a tribasic orthophosphate with a pH of 10.0–12.0 in aqueous solution,
and overall alkalinity aids in its interaction with cell membrane lipids, resulting
in membrane leakage . TSP may also increase
the sensitivity of some microorganisms to nisin and lysozyme.
-
安全性プロファイル
Moderately toxic by intravenous route. Mutation data reported. A strong, caustic material. When heated to decomposition it emits toxic fumes of Na2O and POx. See also PHOSPHATES.