ChemicalBook > CAS DataBase List > Benzyl acetate
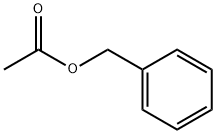
Benzyl acetate
Benzyl acetate
- CAS No.140-11-4
- Chemical Name:Benzyl acetate
- CBNumber:CB8345095
- Molecular Formula:C9H10O2
- Formula Weight:150.17
- MOL File:140-11-4.mol
Benzyl acetate Property
- Melting point -51 °C (lit.)
- Boiling point 206 °C (lit.)
- Density 1.054 g/mL at 25 °C (lit.)
- vapor density 5.1
- vapor pressure 23 mm Hg ( 110 °C)
- refractive index n
20/D 1.502(lit.) - FEMA 2135 | BENZYL ACETATE
- Flash point 216 °F
- storage temp. -20°C
- solubility ≥35.7 mg/mL in EtOH; ≥49.4 mg/mL in DMSO
- form Liquid
- color Colorless liquid
- Odor sweet, floral fruity odor
- explosive limit 0.9-8.4%(V)
- Odor Type floral
- biological source synthetic
- Water Solubility <0.1 g/100 mL at 23 ºC
- Merck 14,1123
- JECFA Number 23
- BRN 1908121
- Exposure limits ACGIH: TWA 10 ppm
- Dielectric constant 5.1(21℃)
- LogP 1.96 at 25℃
- Surface tension 37.3mN/m at 293.15K
- Substances Added to Food (formerly EAFUS) BENZYL ACETATE
- FDA 21 CFR 172.515
- CAS DataBase Reference 140-11-4(CAS DataBase Reference)
- EWG's Food Scores 1
- FDA UNII 0ECG3V79ZJ
- IARC 3 (Vol. 40, Sup 7, 71) 1999
- NIST Chemistry Reference Benzyl ethanoate(140-11-4)
- EPA Substance Registry System Benzyl acetate (140-11-4)
- UNSPSC Code 41116107
- NACRES NA.24
Safety
- Hazard Codes :Xi
- Risk Statements :36/37/38
- Safety Statements :26-37/39-24/25
- RIDADR :2810
- WGK Germany :1
- RTECS :AF5075000
- Autoignition Temperature :862 °F
- TSCA :Yes
- HS Code :29153950
- Hazardous Substances Data :140-11-4(Hazardous Substances Data)
- Toxicity :LD50 orally in rats: 2490 mg/kg (Jenner)
-
NFPA 704:
2 1 0
-
Symbol(GHS)



- Signal wordDanger
- Hazard statements H412
- Precautionary statements P273-P501
Benzyl acetate Price
More Price(16)
- Brand: Sigma-Aldrich(India)
- Product number: W213519
- Product name : Benzyl acetate
- Purity: natural, ≥99%, FCC, FG
- Packaging: 1SAMPLE-K
- Price: ₹5347.55
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: W213519
- Product name : Benzyl acetate
- Purity: natural, ≥99%, FCC, FG
- Packaging: 100G
- Price: ₹16389.05
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: W213519
- Product name : Benzyl acetate
- Purity: natural, ≥99%, FCC, FG
- Packaging: 1KG
- Price: ₹54038.4
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: W213519
- Product name : Benzyl acetate
- Purity: natural, ≥99%, FCC, FG
- Packaging: 5KG
- Price: ₹210091.6
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: W213500
- Product name : Benzyl acetate
- Purity: ≥99%, FCC, FG
- Packaging: 1SAMPLE-K
- Price: ₹5141.88
- Updated: 2022/06/14
- Buy: Buy
Benzyl acetate Chemical Properties,Usage,Production
- Physical properties Benzyl acetate has a characteristic flowery (jasmine) odor and a bitter, pungent taste. It is the main component of jasmine absolute and gardenia oils. It occurs as a minor component in a large number of other essential oils and extracts. It is a colorless liquid with a strong, fruity, jasmine odor. In terms of volume, benzyl acetate is one of the most important fragrance and flavor chemicals. Although benzyl acetate is present in some essential oils at levels up to 65%, most of the commercial product is of synthetic origin.
-
Chemical Properties
Benzyl acetate is a colorless liquid with a fruity odor. On burning and decomposition, it produces irritating fumes. Benzyl acetate reacts with strong oxidants causing fire and explosion hazard.

Benzyl acetate is stable under normal conditions of use. Heating to decomposition may release carbon monoxide, carbon dioxide and other potentially toxic fumes and gases. Vapors may form explosive mixtures with air at elevated temperatures (> 90°C / 194°F). Avoid heat, open flames and other potential sources of ignition.
It has been used as a food additive in fruit flavours and as a component of perfumes since the early 1990s and is widely used as a fragrance in soaps, detergents and incense. There is widespread human exposure to benzyl acetate by ingestion, skin application and inhalation. - Occurrence Present as a main constituent in several oils and flower absolutes: ylang-ylang, cananga, neroli, jasmine, hyacinth, gardenia, tuberose. It has been isolated from the essential oil of the flowers of Loiseleuria procumbens Desv. (azelea). Also reported found in apricot, cooked asparagus, mozzarella cheese, grilled beef, cooked pork, malt whiskey, fresh mango, malt, wort and clams.
- Uses Benzyl acetate is used as an artificial jasmine and other perfumes, soap perfume, flavoring agent, solvent for cellulose acetate and nitrate, natural and synthetic resins, oils, lacquers, polishes, printing inks, and varnish removers.
- Uses Benzyl acetate is produced by reacting benzyl chloride with an alkali acetate or by esterifying benzyl alcohol with acetic anhydride. It is also formed in the oxidation of toluene in the presence of acetic acid or acetic anhydride. Benzyl acetate is one of the most used odorants. It is also used as a flavor and, to a small extent, as a high-boiling solvent.
- Application Benzyl acetate can be used as a high boiling point solvent in coatings, like ink coating, binding agent and paint remover. It is used in soap and other chemical essences and has the effect of promote in floral and fantasy essences in jasmine, white orchid, fragrant plantain lily, gekkakou, narcissus and other essences. It is a plasticizer for ionophore membranes.
- Definition ChEBI: Benzyl acetate is the acetate ester of benzyl alcohol. It has a role as a metabolite. It is an acetate ester and a benzyl ester.
- Preparation Benzyl acetate is produced by the interaction of benzyl chloride and sodiumacetate, by acetylation of benzyl alcohol, or from benzaldehyde and acetic acid with zinc dust.
- Aroma threshold values Detection: 2 to 270 ppb
- Taste threshold values Taste characteristics at 40 ppm: sweet and fruity
-
Synthesis Reference(s)
Chemical and Pharmaceutical Bulletin, 35, p. 1608, 1987 DOI: 10.1248/cpb.35.1608
The Journal of Organic Chemistry, 31, p. 2033, 1966 DOI: 10.1021/jo01344a544
Synthetic Communications, 24, p. 1045, 1994 DOI: 10.1080/00397919408020781 - General Description Colorless liquid with an odor of pears.
- Air & Water Reactions Insoluble in water.
- Reactivity Profile Benzyl acetate is an ester. Esters react with acids to liberate heat along with alcohols and acids. Strong oxidizing acids may cause a vigorous reaction that is sufficiently exothermic to ignite the reaction products. Heat is also generated by the interaction of esters with caustic solutions. Flammable hydrogen is generated by mixing esters with alkali metals and hydrides. Benzyl acetate is incompatible with strong oxidizing agents. Benzyl acetate is also incompatible with acids, bases and reducing agents.
- Health Hazard Exposures to benzyl acetate cause adverse health effects. The symptoms of toxicity and poisoning include irritation to the skin, eyes, burning sensation, confusion, dizziness, drowsiness, labored breathing, sore throat, nausea, vomiting, and diarrhea. Benzyl acetate also causes adverse health effects to the respiratory tract and the CNS system with neurological effects.
- Fire Hazard Benzyl acetate is combustible.
- Flammability and Explosibility Non flammable
- Safety Profile A poison by inhalation.Moderately toxic by ingestion and subcutaneous routes.Human systemic effects by inhalation: an antipsychotic,unspecified respiratory and urinary system effects.Questionable carcinogen with experimental tumorigenicdata. Combusti
- Carcinogenicity Not listed by ACGIH, IARC, NTP, or California Proposition 65.
- Metabolism The esters of benzyl alcohol, such as the acetate, benzoate, cinnamate and hydrocinnamate, are rapidly hydrolysed in vivo to benzyl alcohol which is then oxidized to benzoic acid and excreted as hippuric acid
- storage Benzyl acetate should be kept stored in a cool, dry place with the container closed when not in use.
- Purification Methods Purify the acetate by fractional distillation, preferably in a good vacuum. Values of n25 of 1.5232-1.5242 are too high and should be nearer to 1.4994. [Merker & Scott J Org Chem 26 5180 1961, Beilstein 6 IV 2262.]
- Precautions Exposures to benzyl acetate far above the OEL may result in unconsciousness. After handling and using benzyl acetate, workers should wash thoroughly and remove contaminated clothing, washing it before reuse. Workers should avoid any kind of contact of benzyl acetate with the eyes, skin, ingestion, and inhalation. Workers should wear safety glasses and chemical goggles to avoid splashing of the chemical substance during work, and wear appropriate protective gloves and clothing to prevent skin exposure
Benzyl acetate Preparation Products And Raw materials
Raw materials
Preparation Products
Global(746)Suppliers
- Americas 1 Argentina 1 Australia 2 Belgium 3 Brazil 3 Canada 6 China 441 Egypt 1 Europe 5 France 4 Germany 16 India 100 Ireland 1 Italy 3 Japan 7 Mexico 1 Russia 1 Singapore 1 Slovakia 1 South Africa 2 South Korea 1 Spain 6 Sweden 1 Switzerland 4 Turkey 1 United Kingdom 26 United States 107 Global 746
-
Supplier:
Wuhan Biet Co., Ltd.
- Tel: +8617320528784
- Email:min@biet.com.cn
- Country:China
- ProdList:42
- Advantage:58
-
Supplier:
Hebei Chuanghai Biotechnology Co,.LTD
- Tel: +86-13131129325
- Email:sales1@chuanghaibio.com
- Country:China
- ProdList:5471
- Advantage:58
-
Supplier:
Hebei Kingfiner Technology Development Co.Ltd
- Tel:+86-15532196582<br/>+86-15373005021
- Email:lisa@kingfinertech.com
- Country:China
- ProdList:3005
- Advantage:58
-
Supplier:
Hebei Chuanghai Biotechnology Co., Ltd
- Tel:+86-15531157085;<br/>+8615531157085
- Email:abby@chuanghaibio.com
- Country:China
- ProdList:8805
- Advantage:58
-
Supplier:
Hebei Yanxi Chemical Co., Ltd.
- Tel: +8617531153977
- Email:allison@yan-xi.com
- Country:China
- ProdList:5853
- Advantage:58
-
Supplier:
Henan Bao Enluo International TradeCo.,LTD
- Tel:+86-17331933971<br/>+86-17331933971
- Email:deasea125996@gmail.com
- Country:China
- ProdList:2472
- Advantage:58
-
Supplier:
Hebei Zhuanglai Chemical Trading Co.,Ltd
- Tel: +8613343047651
- Email:admin@zlchemi.com
- Country:China
- ProdList:3692
- Advantage:58
-
Supplier:
Shandong Deshang Chemical Co., Ltd.
- Tel:+86-0531-8875-2665<br/>+8613153039501
- Email:info@deshangchem.com
- Country:China
- ProdList:661
- Advantage:58
-
Supplier:
JINING XINHE CHEMICAL CO., LTD
- Tel: +8615318402391
- Email:sales@xinhepharma.com
- Country:China
- ProdList:809
- Advantage:58
-
Supplier:
Capot Chemical Co.,Ltd.
- Tel:+86-(0)57185586718<br/>+86-13336195806
- Email:sales@capot.com
- Country:China
- ProdList:29730
- Advantage:60
Related articles
140-11-4, Benzyl acetateRelated Search:
- Benzyl benzoate Benzyl nicotinate Vinyl acetate Methyl chloroacetate Benzyl isocyanate Benzyl chloride Methyl bromoacetate Sodium acetate Trimethyl orthoacetate Ethyl 2-(Chlorosulfonyl)acetate Ethyl acetate Methyl 2,2-dimethylphenylacetate Benzyl alcohol Methyl acetate Methyl phenoxyacetate Methyl cyanoacetate PARA METHOXY BENZYL ACETATE Butyl acetate
- bc0001
- organic chemical
- FINE Chemical & INTERMEDIATES
- 医用原料
- 抑制剂
- 其它原料及中间体
- 高端化学
- 大化工产品
- 有机类
- 化工
- 有机产品
- 香料
- 精细化工品
- 香精香料行业
- 通用生化试剂-有机酸
- 单体香料
- 香料食品类
- 化学试剂
- 化工材料
- 医药、农药及染料中间体
- 合成中间体
- 医药原料药
- 化工原料
- 原料
- 日化原料
- 化工产品-有机溶剂
- 对照品
- 酯类
- 原料药
- 有机化工原料
- 化工原料
- 日化
- 日用化学品
- 合成香料
- 脂肪族羧酸酯
- 有机化工
- 有机原料
- 植物提取物
- 分析标准品
- 芳烃
- 天然等同香料和人造香料
- 食用香料(增香剂)
- 食品添加剂
- 食品香料
- 香精香料
- 其他食品检测标准品
- 植物生化提取物
- 农业和环境标准品
- Alphabetic
- Analytical Chromatography Product Catalog
- Analytical Standards
- Building Blocks
- Carbonyl Compounds
- C8 to C9
- BA - BH
- Esters
- Organic Building Blocks
- CH3CO2CH2C6H5