ChemicalBook > CAS DataBase List > Trifluoromethanesulfonic acid
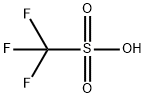
Trifluoromethanesulfonic acid
Trifluoromethanesulfonic acid
- CAS No.1493-13-6
- Chemical Name:Trifluoromethanesulfonic acid
- CBNumber:CB7247316
- Molecular Formula:CHF3O3S
- Formula Weight:150.08
- MOL File:1493-13-6.mol
Trifluoromethanesulfonic acid Property
- Melting point -40 °C
- Boiling point 162 °C (lit.)
- Density 1.696 g/mL at 25 °C (lit.)
- vapor density 5.2 (vs air)
- vapor pressure 8 mm Hg ( 25 °C)
- refractive index n
20/D 1.327(lit.) - RTECS PB2771000
- Flash point None
- storage temp. Store below +30°C.
- solubility Miscible in H<sub>2</sub>O
- pka -14(at 25℃)
- form Fuming Liquid
- Specific Gravity 1.696
- color slightly brown
- PH <1 (H2O)
- Viscosity 1.872mm2/s
- Water Solubility SOLUBLE
- Sensitive Hygroscopic
- Merck 14,9676
- BRN 1812100
- Stability Stable. Incompatible with acids, alkalis, metals.
- InChIKey ITMCEJHCFYSIIV-UHFFFAOYSA-N
- LogP 0.3 at 25℃
- FDA 21 CFR 173.395
- Substances Added to Food (formerly EAFUS) TRIFLUOROMETHANE SULFONIC ACID
- CAS DataBase Reference 1493-13-6(CAS DataBase Reference)
- EWG's Food Scores 2
- FDA UNII JE2SY203E8
- NIST Chemistry Reference CF3SO3H(1493-13-6)
- EPA Substance Registry System Methanesulfonic acid, trifluoro- (1493-13-6)
- UNSPSC Code 12352106
- NACRES NA.22
Safety
- Hazard Codes :C
- Risk Statements :21/22-35-10
- Safety Statements :26-36/37/39-45
- RIDADR :UN 3265 8/PG 2
- WGK Germany :2
- F :3-10
- Hazard Note :Corrosive/Hygroscopic
- TSCA :Yes
- HazardClass :8
- PackingGroup :II
- HS Code :29049020
- Toxicity :LD50 orally in Rabbit: 1605 mg/kg LD50 dermal Rat > 2000 mg/kg
-
NFPA 704:
0 3 1
-
Symbol(GHS)


- Signal wordDanger
- Hazard statements H290-H302-H314-H335
- Precautionary statements P234-P261-P280-P301+P312-P303+P361+P353-P305+P351+P338
Trifluoromethanesulfonic acid Price
More Price(14)
- Brand: Sigma-Aldrich(India)
- Product number: 347817
- Product name : Trifluoromethanesulfonic acid
- Purity: ReagentPlus?, ≥99%
- Packaging: 5G
- Price: ₹6910.25
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: 8.21166
- Product name : Trifluoromethanesulfonic acid
- Purity: for synthesis
- Packaging: 100ML
- Price: ₹6990
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: 347817
- Product name : Trifluoromethanesulfonic acid
- Purity: ReagentPlus?, ≥99%
- Packaging: 25G
- Price: ₹23399.9
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: 8.21166
- Product name : Trifluoromethanesulfonic acid
- Purity: for synthesis
- Packaging: 1L
- Price: ₹45380
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: 347817
- Product name : Trifluoromethanesulfonic acid
- Purity: ReagentPlus?, ≥99%
- Packaging: 100G
- Price: ₹39056.1
- Updated: 2022/06/14
- Buy: Buy
Trifluoromethanesulfonic acid Chemical Properties,Usage,Production
- Description Trifluoromethanesulfonic acid, also known as triflic acid, TFMS, TFSA, HOTf or TfOH, is a sulfonic acid with the chemical formula CF3SO3H. It is often regarded as one of the strongest acids, and is one of a number of so-called "superacids". it is used in the manufacture of pharmaceuticals, agricultural chemicals and polymers. The anhydrous form is widely used in fine chemical synthesis. It is non-oxidizing, has a high thermal stability, and is resistance to both oxidation and reduction, which makes it one of the more useful compounds in the super acids class. In the pharma industry, it is used to make a number of drug classes, including nucleosides, antibiotics, steroids, proteins and glycosides. Triflic anhydride reacts readily with water and has an unfavorable toxicity profile.
-
Chemical Properties
Trifluoromethanesulfonic acid is a hygroscopic, colorless liquid at room temperature. It is soluble in polar solvents such as dimethylformamide (DMF), dimethyl sulfoxide (DMSO), acetonitrile, and dimethyl sulfone. Addition of triflic acid to polar solvents can be dangerously exothermic.
Trifluoromethanesulfonic acid is widely used especially as a catalyst and a precursor in organic chemistry. With an Ka = 8. 0 1014 (pKa ~ -15) mol/kg, HOTf qualifies as a superacid. Triflic acid owes many of its useful properties to its great thermal and chemical stability. Both the acid and its conjugate base CF3SO3-, known as triflate, resist oxidation/reduction reactions, whereas many strong acids are oxidizing, e. g. HClO4 and HNO3. The triflate anion is immune to attack by even strong nucleophiles. Because of its resistance to oxidation and reduction, triflic acid is a very useful and versatile reagent. Further recommending its use, triflic acid does not sulfonate substrates, which can be a problem with sulfuric acid, fluorosulfuric acid, and chlorosulfonic acid. Below is a prototypical sulfonation, which HOTf does not undergo: C6H6 + H2SO4 → C6H5(SO3H) + H2O. - Preparation Yellow-brown liquid. The boiling point is 167~170 ℃.The refractive index is 1.331.The relative density is 1.708.It is the strongest organic acids, easily soluble in water.Use carbon disulfide as raw material, with the reaction of iodine pentafluoride to produce trifluoromethyl disulfide.(CF3S) 2Hg was obtained when reacting with mercury; Then through oxidation of hydrogen oxide, trifluoromethanesulfonic acid is acquired.
-
Uses
- It is used for organic synthesis, widely used in pharmaceutical and chemical industries, such as nucleosides, antibiotics, steroids, protein, sugar, vitamins synthesis, silicone rubber modification.
- Isomerization and alkylation of the catalyst, the preparation of 2, 3-dihydro-2-indanone, tetralone, glycosides in the removal of glycoproteins.
-
Reactions
Trifluoromethanesulfonic acid acts as a catalyst for esterification reactions and an acidic titrant in nonaqueous acid-base titration. It is useful in protonations due to the presence of conjugate base triflate is non nucleophilic. It serves as a deglycosylation agent for glycoproteins. In addition, it is a precursor and a catalyst in organic chemistry. It reacts with acyl halides to prepare mixed triflate anhydrides, which are strong acylating agents used in Friedel-Crafts reactions. It acts as a key starting material for the preparation of ethers and olefins by reacting with alcohols as well as to prepare trifluoromethanesulfonic anhydride by dehydration reaction.
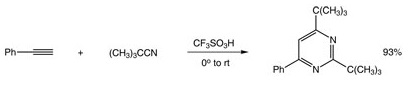
Catalyst used in the production of cocoa butter substitute from palm oil. This is a very similar reaction to what would be done if one wanted to create polymers using triflic acid in the synthesis. Other Friedel-Crafts type reactions using triflic acid include cracking of alkanes and alkylation of alkenes which are very important to the petroleum industry. These triflic acid derivative catalysts are very effective in isomerizing straight chain or slightly branched hydrocarbons that can increase the octane rating of a particular petroleum based fuel. - Uses Trifluoromethanesulfonic acid acts as a catalyst in Friedel-Crafts type acylation, alkylation and polymerization reactions; as a solvent for ESR; as a nonaqueous strong acid titrant; with trifluoroacetic acid, q.v., in solid-phase peptide synthesis. One of the strongest available monoprotic acids.
- Definition ChEBI: Trifluoromethanesulfonic acid is a one-carbon compound that is methanesulfonic acid in which the hydrogens attached to the methyl carbon have been replaced by fluorines. It is a one-carbon compound and a perfluoroalkanesulfonic acid. It is a conjugate acid of a triflate.
- General Description Trifluoromethanesulfonic acid is a strong organic acid. It can be prepared by reacting bis(trifluoromethylthio)mercury with H2O2. On mixing with HNO3, it affords a nitrating reagent (a nitronium salt). This reagent is useful for the nitration of aromatic compounds. Its dissociation in various organic solvents has been studied.
-
reaction suitability
reaction type: Acid-base reactions
reaction type: Redox Reactions - Safety Profile A corrosive irritant to the skin, eyes, and mucous membranes. A strong acid. Violent reaction with acyl chlorides or aromatic hydrocarbons evolves toxic hydrogen chloride gas. When heated to decomposition it emits toxic fumes of Fand SOx. See also FLUORIDES.
Trifluoromethanesulfonic acid Preparation Products And Raw materials
Raw materials
Preparation Products
Global(740)Suppliers
-
Supplier:
JIANGXI TIME PHARMACEUTICAL CO.,LTD
- Tel:+86-0794-7183888<br/>+86-13155888520
- Email:sales@groupchem.com
- Country:China
- ProdList:27
- Advantage:58
-
Supplier:
Dalian Richfortune Chemicals Co., Ltd
- Tel:0411-84820922<br/>8613904096939
- Email:sales@richfortunechem.com
- Country:China
- ProdList:304
- Advantage:57
-
Supplier:
CD Chemical Group Limited
- Tel: +8615986615575
- Email:info@codchem.com
- Country:China
- ProdList:20342
- Advantage:58
-
Supplier:
Shandong Believe Chemical PTE.LTD
- Tel:+86-18553607090<br/>+8618553607090
- Email:1093908296@qq.com
- Country:China
- ProdList:1667
- Advantage:58
-
Supplier:
Henan Allgreen Chemical Co.,LTD
- Tel:+86-37155567971<br/>+86-13633837469
- Email:info@allgreenchem.com
- Country:China
- ProdList:5998
- Advantage:58
-
Supplier:
CHANGZHOU HARVECHEM LTD
- Tel:+86-0519-88784888<br/>+8613906120163
- Email:inquiry@harvechem.com
- Country:China
- ProdList:284
- Advantage:58
-
Supplier:
Hebei Chuanghai Biotechnology Co., Ltd
- Tel:+86-15531157085<br/>+86-15531157085
- Email:abby@chuanghaibio.com
- Country:China
- ProdList:8808
- Advantage:58
-
Supplier:
Hebei Chuanghai Biotechnology Co,.LTD
- Tel: +86-13131129325
- Email:sales1@chuanghaibio.com
- Country:China
- ProdList:5870
- Advantage:58
-
Supplier:
airuikechemical co., ltd.
- Tel:+86-18353166132<br/>+86-18353166132
- Email:sales02@airuikechemical.com
- Country:China
- ProdList:983
- Advantage:58
-
Supplier:
Hebei Zhuanglai Chemical Trading Co.,Ltd
- Tel: +8613343047651
- Email:admin@zlchemi.com
- Country:China
- ProdList:3692
- Advantage:58
Related articles
1493-13-6, Trifluoromethanesulfonic acidRelated Search:
- Methyl trifluoromethanesulfonate Pefloxacin mesylate Citric acid (Trifluoromethoxy)benzene TRIFLUOROMETHANESULFONYL FLUORIDE Stearic acid Scandium trifluoromethanesulfonate Trifluoromethanesulfonic anhydride Hyaluronic acid Methanesulfonic acid Trifluoroacetic acid Benzotrifluoride Ethyl 2-(Chlorosulfonyl)acetate Folic acid Levofloxacin Methylate Sodium trifluoromethanesulfonate λ-Cyhalothrin Stannous methanesulfonate Triflic Acid
- YP00036
- 1493-13-6
- Pharmaceutical intermediates
- straight chain compounds
- Volumetric Solutions
- Titration
- Synthetic Reagents
- Solutions for non-aqueous titrations
- By Reference Material
- Acid SolutionsChemical Synthesis
- Reference Material Potassium hydrogenphthalateTitration
- Organic AcidsVolumetric Solutions
- organofluorine compounds
- Organic Fluorides
- 日用化工
- 医用原料
- 有机合成中间体
- 生物化工
- 羧酸
- 其他化工产品
- 化学试剂
- 氟化工
- 其他
- 合成材料中间体
- 高端化学
- 有机类
- 其他危险化学品
- 其它化工品
- 大化工产品
- 有机合成催化剂
- 原料
- 化学试剂-有机试剂
- 化工
- 水处理化工原料
- 有机产品
- 化学产品
- 化工产品-有机化工
- 原料药
- 精细化工品
- 化工材料
- 化工原料
- 医药、农药及染料中间体
- 医药原料和中间体
- 其他原料
- 化学药物
- 有机化工原料
- 其他中间体
- 化工中间体
- 医药中间体
- 有机中间体
- 标准品
- 有机砌块
- 磺酸/亚磺酸盐
- 氟化物
- 医药原料
- 催化剂
- 有机酸
- 医用溶剂