How to synthesize Trifluoromethanesulfonic acid?
Description
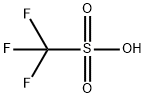
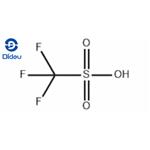
Triflic acid (Trifluoromethanesulfonic acid) is a sulfonic acid with the chemical formula CF3SO3H. It is one of the strongest acids and is mainly used in research as a catalyst for esterification[1]. Triflic acid is a hygroscopic, colorless liquid at room temperature. It is soluble in polar solvents such as DMF, DMSO, acetonitrile, and dimethyl sulfone. Triflic acid is a nonoxidizing, thermally stable compound resistant to oxidation and reduction. In the laboratory, triflic acid is helpful in protonations because the conjugate base of triflic acid is non-nucleophilic. Triflic acid promotes other Friedel-Crafts-like reactions, including the cracking of alkanes and alkylation of alkenes, which are very important to the petroleum industry[2].
Chemical Property
Trifluoromethanesulfonic acid has often been acclaimed as the strongest of all known monoprotic organic acids. Fluorosulfonic acid has also been given the same status. Trifluoromethanesulfonic acid and its conjugate base have extreme thermal stability and resistance to reductive and oxidative cleavage. They do not provide a source of fluoride ions, even in the presence of strong nucleophiles. The nonoxidizing nature of trifluoromethanesulfonic acid can be beneficial in minimizing or eliminating side reactions in some instances, and it reduces the hazards associated with strong oxidizing acids such as perchloric acid.
Pure trifluoromethanesulfonic acid is a clear, colorless liquid that boils at 162 ℃ (760 Torr). By comparison, methanesulfonic acid boils at 165 ℃ (8.5 Torr), reflecting a much higher intermolecular association. Trifluoromethanesulfonic acid fumes in moist air until it is converted to a stable monohydrate, which is a solid at room temperature (mp 34 ℃). The monohydrate is more correctly termed hydronium trifluoromethanesulfonate since water is quite a good base in such a strong acid. Like the analogous hydronium perchlorate, this salt is very hygroscopic and will liquefy upon contact with a moist atmosphere. An x-ray crystallographic study showed that its structure comprises oxonium ions, which are hydrogen-bonded to three sulfonate groups in a pyramidal arrangement.
Synthesis method
The first reported syntheses of trifluoromethanesulfonic acid appeared in 1954. Haszeldine and Kidd obtained the acid by oxidation of bis(trifluoromethy1thio)mercury with aqueous hydrogen peroxide. This process was later modified by the direct formation of bis- (trifluoromethy1thio)mercury from carbon disulfide and mercuric fluoride. Haszeldine and Kidd have also described an alternative route to the acid via trifluoromethane sulfonyl chloride. Commonly, the hygroscopic salts of trifluoromethanesulfonic acid are dehydrated at about 100 ℃ under vacuum before adding sulfuric acid[3].
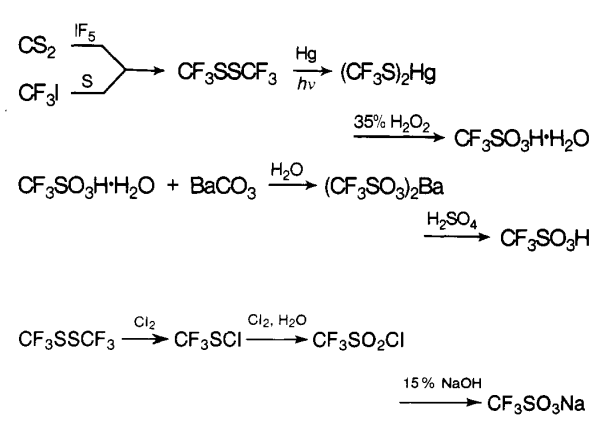
Methyltrifluoromethyl sulfide is another intermediate that has been employed in the synthesis of trifluoromethanesulfonic acid. The sulfide is most conveniently prepared by the reaction of trifluoroiodomethane with sodium methanethiolate, but even higher yields are obtained from a photochemical reaction. The oxidation of methyl trifluoromethyl sulfide to the sulfonate salt was successfully carried out under various conditions.
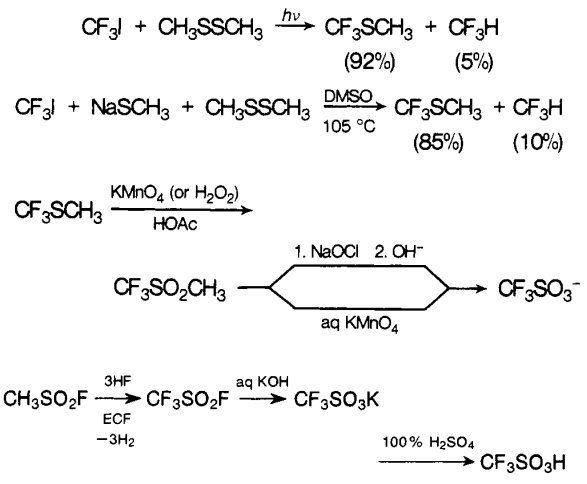
The disclosure that trifluoromethanesulfonic acid, as well as higher homologous perfluoroalkanesulfonic acids, can be prepared by electrochemical fluorination (ECF) of alkanesulfonyl fluorides (or chlorides) was also made in 1954.
References
[1] Philip A. Staniland. “29-Poly(ether ketone)s.”Comprehensive Polymer Science and Supplements 5 (1989): 483-497.
[2] Keki Hormusji Gharda. “A process for the manufacture of triflic acid.”2011.
[3] Sudip Mukhopadhyay. “Synthesis of Trifluoromethanesulfonic Acid from CHF3.” Organic Process Research & Development 8 4 (2004): 660–662.
You may like
Related articles And Qustion
See also
Lastest Price from Trifluoromethanesulfonic acid manufacturers
US $0.00-0.00/KG2025-11-18
- CAS:
- 1493-13-6
- Min. Order:
- 1KG
- Purity:
- 98
- Supply Ability:
- 10000KGS

US $0.00/KG2025-11-13
- CAS:
- 1493-13-6
- Min. Order:
- 50KG
- Purity:
- 99%
- Supply Ability:
- 10tons