Trifluoromethanesulfonic acid: Applications and Synthesis
Introduction
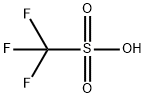
Trifluoromethanesulfonic acid (TfOH)[1], also known as triflic acid, is a strong organic acid with the chemical formula CF3SO3H. It is a colorless liquid that is soluble in water and many organic solvents. TfOH is one of the strongest acids currently known, with a acidity constant (pKa) of -14, which makes it about 1 million times stronger than sulfuric acid. Due to its high acidity and ability to act as an efficient catalyst, TfOH finds wide use in various organic synthesis reactions such as esterification, alkylation, and polymerization. It is also used as a dehydrating agent, desiccant and as a catalyst in a variety of industrial processes. However, due to its corrosive nature and toxicity, TfOH must be handled with caution and proper protective measures should be taken when working with it.

Figure 1 appearance of Trifluoromethanesulfonic acid
Application
Trifluoromethanesulfonic acid is commonly used as a catalyst in esterification reactions. For example, it can be used to convert alcohols into their corresponding esters, which have various applications in the fragrance and flavor industries.
It polymerizes initiator for certain monomers, such as styrene and vinyl acetate. This leads to the formation of high molecular weight polymers with desirable properties, such as toughness and flexibility. Trifluoromethanesulfonic acid can be used as a catalyst for Friedel-Crafts reactionsit can promote the alkylation or acylation of substituted benzene rings.
Researchers usually remove water from various polar compounds with it. For example, it can be used to convert alcohols into their corresponding ethers or alkyl halides[2,3]. It is a highly effective etchant for silicon dioxide, making it useful in the electronics industry for the production of microelectronic devices. Its versatility and effectiveness make it a valuable tool in many areas of chemistry and industry[4].
Trifluoromethanesulfonic acid (TfOH) was used to catalyze the alkylation of isobutane with 2-butene. TfOH can appropriately increase the ratio of catalyst B/L acid sites, which is beneficial to improve the selectivity of alkylation products. When the amount of TfOH is 20% of the mass of the carrier, the catalytic effect is the best. Under the optimal conditions of reaction temperature of 80°C, reaction pressure of 2.5 MPa, and olefin mass space velocity of 0.8 h~(-1), the catalyst had a longer lifetime and higher conversion of olefins, and the selectivity of C8 products remained at 65 % above[5].
toxicity
Trifluoromethanesulfonic acid (TFMSA) is a highly corrosive and toxic compound. It is a strong acid that can cause severe skin and eye irritation, and inhalation or ingestion of the vapor or liquid can lead to serious health problems. TFMSA is also an oxidizing agent, which means it can react violently with other substances, including flammable materials. This makes it extremely hazardous to handle, and it requires careful storage and disposal procedures. In summary, TFMSA is a highly toxic and corrosive substance that poses significant health and safety risks to those who handle it. It should only be used by trained professionals in properly equipped facilities under strict safety protocols.
Synthesis
Trifluoromethanesulfonic acid, also known as triflic acid, can be synthesized by reacting chlorosulfonic acid with hydrogen fluoride. The reaction is highly exothermic and must be conducted with caution under controlled conditions.
Trifluoromethanesulfonic acid (TFMSA) can be synthesized by two methods:
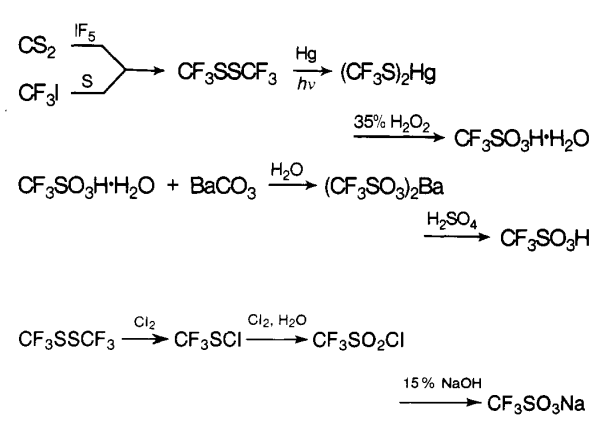
Under hydrogen fluoride atmosphere, DMF-DMA was obtained by oxidation of trifluoromethanethiol. The chemical equation is shown in Figure 2.
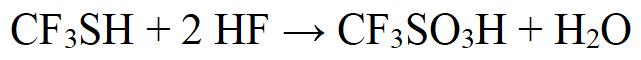
Figure 2 chemical equation of Trifluoromethanesulfonic acid
Using sulfur hexafluoride as a catalyst, sodium trifluoromethanesulfonate reacts with bromoethane to obtain DMF-DMA. The chemical equation is shown in Figure 3.
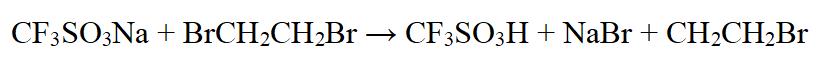
Figure 3 chemical equation of Trifluoromethanesulfonic acid
It is important to note that these reaction processes require special operating conditions and safety precautions, as TFMSA is a highly corrosive and toxic substance. Strict safety standards and protocols must be followed when conducting any experiments or preparation processes involving TFMSA.
References
[1] Rakita P, Triflic acid and its derivatives: A family of useful reagents for synthesis [J]. Chimica Oggi, 2004, 22: 48-50.
[2] Takahashi Y, Ito H, Machida K, et al. Regioselective Deprotection of Alkoxy Groups with Trifluoromethanesulfonic Acid in the Presence of Fluoride Ions. J. Org. Chem. 2014, 79 (1), 337-344.
[3] Jin QN, Liang XG, Han, JF, et al. Trifluoromethanesulfonic Acid-Promoted Three-Component Cyclization of Alkynes, Aldehydes, and Amines for the Synthesis of 3-Amino Furans and Pyrroles. Chem. Eur. J. 2018, 24 (9), 2097-2102.
[4]Wang B, Liu X, Zhang L, et al. Triflic Acid-Catalyzed Reaction of Ethers and Alcohols: Facile Synthesis of Tetrahydrofurans and 1,3-Dioxanes. Angew. Chem. Int. Ed. 2018, 57 (28), 8425-8429.
[5] Zhang Shizhong, Yu Fengli, Wang Zhiping, Xie Congxia. The alkylation reaction of isobutane/2-butene catalyzed by trifluoromethanesulfonic acid supported on micro-mesoporous Hβ zeolite[J]. Journal of Qingdao University of Science and Technology (Natural Science Edition), 2021, 42(06):21-27.
Related articles And Qustion
See also
Lastest Price from Trifluoromethanesulfonic acid manufacturers
US $0.00-0.00/KG2025-11-18
- CAS:
- 1493-13-6
- Min. Order:
- 1KG
- Purity:
- 98
- Supply Ability:
- 10000KGS

US $0.00/KG2025-11-13
- CAS:
- 1493-13-6
- Min. Order:
- 50KG
- Purity:
- 99%
- Supply Ability:
- 10tons