ChemicalBook > CAS DataBase List > cefsulodin sodium salt
cefsulodin sodium salt
Cephalosporins Category Toxicity grading Acute toxicity Flammability and hazard characteristics Storage Characteristics Extinguishing agent
cefsulodin sodium salt
- CAS No.52152-93-9
- Chemical Name:cefsulodin sodium salt
- CBNumber:CB5270251
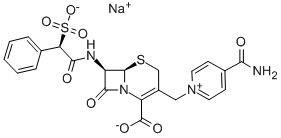
- Molecular Formula:C22H19N4O8S2.Na
- Formula Weight:554.53
- MOL File:52152-93-9.mol
cefsulodin sodium salt Property
- Melting point 175 C
- storage temp. Inert atmosphere,Store in freezer, under -20°C
- solubility H2O: 50 mg/mL, clear, light yellow
- form powder or crystals
- color Off-White to Pale Yellow
- Water Solubility Soluble in water.
- Merck 13,1958
- Stability Hygroscopic
- InChIKey REACMANCWHKJSM-DWBVFMGKSA-M
- FDA UNII 2D087186PY
- EPA Substance Registry System Pyridinium, 4-(aminocarbonyl)-1-[[(6R,7R)-2-carboxy-8-oxo-7-[[(2R)-phenylsulfoacetyl]amino]-5-thia-1-azabicyclo[4.2.0]oct-2-en-3-yl]methyl]-, inner salt, monosodium salt (52152-93-9)
Safety
- Hazard Codes :Xi,Xn
- Risk Statements :36/37/38-42/43-20/21/22
- Safety Statements :22-26-36/37-45-36
- WGK Germany :2
- RTECS :UU1785000
- TSCA :Yes
- HS Code :29419059
- Toxicity :LD50 in mice (mg/kg): >4000 i.p.; >15000 orally (Bryskier)
-
Symbol(GHS)


- Signal wordWarning
- Hazard statements H315-H317-H319-H335-H334
- Precautionary statements P261-P280-P305+P351+P338-P280g-P284-P304+P340-P342+P311a-P501a
cefsulodin sodium salt Price
More Price(5)
- Brand: Sigma-Aldrich(India)
- Product number: C8145
- Product name : Cefsulodin sodium salt hydrate
- Purity: third-generation cephalosporin antibiotic
- Packaging: 100MG
- Price: ₹6018.7
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: C8145
- Product name : Cefsulodin sodium salt hydrate
- Purity: third-generation cephalosporin antibiotic
- Packaging: 250MG
- Price: ₹8660
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: C8145
- Product name : Cefsulodin sodium salt hydrate
- Purity: third-generation cephalosporin antibiotic
- Packaging: 1G
- Price: ₹22321.15
- Updated: 2022/06/14
- Buy: Buy
- Brand: SRL
- Product number: 80223
- Product name : Cefsulodin Sodium Salt (CFS) extrapure, 97%
- Purity:
- Packaging: 10mg
- Price: ₹3120
- Updated: 2022/05/26
- Buy: Buy
- Brand: SRL
- Product number: 80223
- Product name : Cefsulodin Sodium Salt (CFS) extrapure, 97%
- Purity:
- Packaging: 250mg
- Price: ₹7490
- Updated: 2022/05/26
- Buy: Buy
cefsulodin sodium salt Chemical Properties,Usage,Production
-
Cephalosporins
Cefsulodine sodium is a cephalosporin antibiotic, its antimicrobial spectrum is narrow, because it has good permeability to the outer layer of the cell wall of Pseudomonas aeruginosa , and it has a high affinity on penicillin-binding protein LA, LB and Ⅲ and thus it has strong bactericidal inhibition effect on the formation of the cell wall peptidoglycan , it has a strong and specific bactericidal effect on P. aeruginosa , the MIC is 1.5μg/ml, and it has high stability on β-lactamase produced by itself . Its antimicrobial activity against Pseudomonas aeruginosa is stronger than cefoperazone, methyl benzyl amoxicillin (10 times), carbenicillin, piperacillin and hydroxyl ampicillin (16 to 32-fold) , and its antimicrobial activity against Pseudomonas aeruginosa is equal to aminoglycoside antibiotics such as gentamicin, dibekacin . And there is no cross-resistance.After intramuscular or intravenous administration 0.5g, 15min respectively ,it achieves the maximum blood concentration of 37.2μg/ml, and the concentration of the drug increases with the increase of the dose . It can be quickly distributed to the kidneys, blood plasma, lung, heart, digestive tract, liver and spleen, and it can go access to sputum, wound exudate, amniotic fluid and breast milk. PBP is 70%, t1/2 is 1.5h, it is mainly excreted in the urine, 6h after treatment the urine excretion rate is 60% to 70%, metabolites with antibacterial activity are not found in the urine . In renal dysfunction patients , plasma concentration increases, half-life extends and urinary excretion rate decreases.
Sodium cefsulodin is clinically used for the treatment of respiratory tract infections caused by Pseudomonas aeruginosa, pyelonephritis, cystitis, peritonitis, septicemia, prostatitis, burns, wounds and otitis media secondary infection, corneal ulcers, especially penicillin and aminoglycosides antibiotic treatment ineffective Pseudomonas aeruginosa infection.
Adverse reactions occurring rate is approximately 1.3%, it is similar to other cephalosporins. Occasionally allergic reactions occur, such as rash, urticaria.There are gastrointestinal reactions. Injection site is painful, intravenous injection causes phlebitis, mildly elevated serum transaminases , few visible proteinuria; there are also visible thrombocytopenia, increased eosinophils; rare thrombocytopenia, vitamin deficiency and the like.
Patients allergic to the chemicalsare are banned .Patients allergic to penicillin,and cephalosporin , having history of allergy diseases, allergic bodies, severe renal dysfunction, and pregnant women should use with caution. Cefsulodine sodium together with diuretics, aminoglycosides can increase renal toxicity. Check liver function, kidney function and blood at regular intervals .
The above information is edited by the chemicalbook of Tian Ye. - Category Toxic substances
- Toxicity grading Low toxicity
- Acute toxicity Intravenous-rat LD50: 3030 mg/kg; Intravenous-Mouse LD50: 3780 mg/kg.
- Flammability and hazard characteristics Thermal decomposition produces toxic nitrogen oxides, sulfur oxides, sodium oxide fumes.
- Storage Characteristics Ventilated, low-temperature ,dry storeroom.
- Extinguishing agent Water, carbon dioxide, foam, powder.
- Description Cefsulodin was synthesized by Takeda Chemicals Industries in 1974 by introducing the sulfobenzyl group, the same moiety as in sulbenicillin, at the 7 position of the cephem nucleus. Its side chain at the 3 position is similar to that of cephaloridine except for the carbamoyl group. The introduction of these hydrophilic groups increases the activity against Pseudomonas aeruginosa, but it markedly decreases it against gram-positive and other gramnegative bacteria. Therefore cefsulodin is used as a specific antibiotic against infections caused by the opportunistic pathogen P. aeruginosa.
- Originator Pseudomonil,Ciba Geigy,W. Germany,1980
- Uses Cefsulodin is most commonly used in CIN agar to select for Yersinia microorganisms. The compound displays a mechanism of action like many β lactam antibiotics through inhibition of cell wall synthesis by competitively inhibiting penicillin binding protein (PBP) crosslinking of peptidoglycan resulting in inhibition of the final transpeptidation step. Through the inability for Cefsulodin sodium salt hydrate to inhibit cefsulodin-resistant mutants of Pseudomonas aeruginosa PAO4089 growth displayed that Cefsulodin sodium salt hydrate may compete with PBP3 in addition to PBP1A and PBP1B.
- Uses A β lactam antibiotic.
- Uses Cefsulodin is a third generation cephalosporin (C258750) antibiotic designed specifically for Pseudomonas Aeruginosa.
- Uses betaadrenergic blocker
- Definition ChEBI: Cefsulodin sodium is an organic molecular entity.
- Manufacturing Process 0.514 g (4 x 10-3 mol) of 7-(α-sulfophenylacetamido)cephalosporanic acid, 0.466 g (3 x 10-3 mol) of isonicotinamide and 2.0 g (2.06 x 10-3 mol) of potassium thiocyanate were dissolved in 2.5 ml of water. The resulting solution was allowed to stand and heated for 20 hours in a thermostat kept at 50°C and then directly purified by chromatography on an Amberlite XAD-2 column (16 x 880 mm). Subsequently, the fractions containing the cephalosporins were collected and subjected to freeze-drying to obtain 270 g of the title product in the form of a pale yellowish white powder. The product is usually used as the sodium salt.
- brand name Cefomonil (TAP).
- Therapeutic Function Antibiotic
- Biological Activity cefsulodin, formerly named as sce-129, is a cephalosporin with a spectrum of antibacterial activity against staphylococcus aureus, pseudomonas aeruginosa, and most other gram-positive cocci [1]. cefsulodin shows little activity against other species of acinetobacter spp., pseudomonas, or members of the enterobacteriaceae [1]. cefsulodin is a β-lactam antibiotic that involved in lysing actively-growing e. coli by specifically binding to the intermembrane proteins, penicillin-binding proteins 1a and b, whose transglycosylase and transpeptidase activities are involved in cell elongation and septation [2].cefsulodin was active against p. aeruginos. cefsulodin was active against penicillinase-producing strains of s. aureus. the mics of cefsulodin for pseudomonas aeruginosa and its mutants pseudomonas aeruginosa pao4089 were 0·78 and 12· mg/l [3]. cefsulodin was active in minimum inhibitory concentrations (mics) of 0.5 to 64 μg/ml. cefsulodin was active against p. diminuta, p. maltophilia, p. paucimobilis, and p. pseudoalcaligenes with mics of 1-32 μg/ml. cefsulodin was not hydrolyzed by the β-lactamase induced in p. aeruginosa by growth in the presence of benzylpenicillin and was a poor substrate for β-lactamases from enterobacter cloacae and proteus morganii [4].
-
References
[1] barry a l, jones r n, thornsberry c. cefsulodin: antibacterial activity and tentative interpretive zone standards for the disk susceptibility test[j]. antimicrobial agents and chemotherapy, 1981, 20(4): 525-529.
[2] jacoby g h, young k d. cell cycle-independent lysis of escherichia coli by cefsulodin, an inhibitor of penicillin-binding proteins 1a and 1b[j]. journal of bacteriology, 1991, 173(1): 1-5.
[3] bryan l e, kwan s, godfrey a j. resistance of pseudomonas aeruginosa mutants with altered control of chromosomal beta-lactamase to piperacillin, ceftazidime, and cefsulodin[j]. antimicrobial agents and chemotherapy, 1984, 25(3): 382-384.
[4] king a, shannon k, phillips i. in vitro antibacterial activity and susceptibility of cefsulodin, an antipseudomonal cephalosporin, to beta-lactamases[j]. antimicrobial agents and chemotherapy, 1980, 17(2): 165-169.
cefsulodin sodium salt Preparation Products And Raw materials
Raw materials
Preparation Products
Global(201)Suppliers
-
Supplier:
Shaanxi Xianhe Biotech Co., Ltd
- Tel: +8617709210191
- Email:Jerry@xhobio.com
- Country:China
- ProdList:884
- Advantage:58
-
Supplier:
Henan Tianfu Chemical Co.,Ltd.
- Tel:+86-0371-55170693<br/>+86-19937530512
- Email:info@tianfuchem.com
- Country:China
- ProdList:21628
- Advantage:55
-
Supplier:
career henan chemical co
- Tel:+86-0371-86658258<br/>+8613203830695
- Email:sales@coreychem.com
- Country:China
- ProdList:29858
- Advantage:58
-
Supplier:
Hubei Jusheng Technology Co.,Ltd.
- Tel:18871490254
- Email:linda@hubeijusheng.com
- Country:CHINA
- ProdList:28172
- Advantage:58
-
Supplier:
BOC Sciences
- Tel:+1-631-485-4226
- Email:inquiry@bocsci.com
- Country:United States
- ProdList:19552
- Advantage:58
-
Supplier:
CONIER CHEM AND PHARMA LIMITED
- Tel: +8618523575427
- Email:sales@conier.com
- Country:China
- ProdList:49732
- Advantage:58
-
Supplier:
SIMAGCHEM CORP
- Tel: +86-13806087780
- Email:sale@simagchem.com
- Country:China
- ProdList:17365
- Advantage:58
-
Supplier:
TargetMol Chemicals Inc.
- Tel:+1-781-999-5354<br/>+1-00000000000
- Email:marketing@targetmol.com
- Country:United States
- ProdList:32159
- Advantage:58
-
Supplier:
Hubei Ipure Biology Co., Ltd
- Tel: +8613367258412
- Email:ada@ipurechemical.com
- Country:China
- ProdList:10244
- Advantage:58
-
Supplier:
Career Henan Chemica Co
- Tel:+86-0371-86658258<br/>+8613203830695
- Email:laboratory@coreychem.com
- Country:China
- ProdList:30231
- Advantage:58
52152-93-9, cefsulodin sodium saltRelated Search:
- Spectrum of Activity
- Penicillins and Cephalosporins (beta-Lactams)
- Mechanism of Action
- Interferes with Cell Wall SynthesisAntibiotics
- Core Bioreagents
- Chemical Structure Class
- AntibioticsAntibiotics
- Antibiotics A-FAntibiotics
- Antibiotics A to
- Antibacterial
- A - KResearch Essentials
- antibiotic
- GUBERNAL
- 抑制剂
- 生化试剂
- 试剂
- 标准品
- 化学试剂
- 有机中间体
- 化工原料
- 原料药及中间体
- 医药化工类
- 生化试剂-氨基酸类
- 医药原料
- 合成
- 小分子抑制剂
- 原料
- 医药中间体
- 医药原料药
- 原料药
- Antibiotics A to Z
- Antibiotics A-F
- Antibiotics
- BioChemical
- C22H19N4O8S2NaH2O
- C22H19N4O8S2Na
- C22H20N408S2
- C22H19N4NaO8S2
- 头孢磺啶钠,10 MM DMSO 溶液
- 头孢磺啶钠(中间体)
- (6R,7R)-3-((4-氨基甲酰基吡啶-1-鎓-1-基)甲基)-8-氧代-7-((R)-2-苯基-2-磺酰基乙酰氨基)-5-硫杂-1-氮杂双环[4.2.0]辛-2-烯-2-羧酸钠盐
- CEFSULODIN SODIUM SALT HYDRATE 头孢磺吡苄钠盐水合物
- 头孢磺啶钠水合物
- 头孢磺啶钠(痢菌净(乙酰甲喹))
- 头孢磺啶钠(第三代头孢菌素)
- 痢菌净(乙酰甲喹)
- 磺吡苄头孢菌素钠
- 头孢磺吡啶钠
- 头孢磺吡苄钠
- 达克舒林
- 头孢磺啶钠
- 头孢磺啶钠盐
- (6R,7R)-3-[(4-氨基甲酰基吡啶-1--1-基)甲基]-8-氧代-7-[[(2R)-2-苯基-2-磺酸基乙酰]氨基]-5-硫-1-氮杂双环[4.2.0]辛-2-烯-2-甲酸钠
- 钠(6R,7R)-3-((4-胺甲酰基吡啶鎓-1-基)甲基)-8-氧代-7-((R)-2-苯基-2-磺酸盐基乙酰胺基)-5-硫代-1-氮杂双环[4.2.0]辛-2-烯-2-甲酸酯
- 头孢磺吡苄钠盐
- 头孢罗啶钠
- 头孢磺啶钠盐水合物
- 头孢磺吡苄钠盐水合物