ChemicalBook > CAS DataBase List > Diquafosol Tetrasodium
Diquafosol Tetrasodium
Diquafosol Tetrasodium
- CAS No.211427-08-6
- Chemical Name:Diquafosol Tetrasodium
- CBNumber:CB41347117
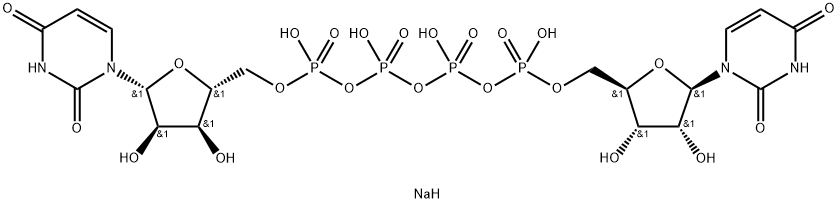
- Molecular Formula:C18H27N4NaO23P4
- Formula Weight:814.3
- MOL File:211427-08-6.mol
Diquafosol Tetrasodium Property
- storage temp. Store at -20°C
- solubility DMSO:1.0(Max Conc. mg/mL);1.14(Max Conc. mM)
Water:100.0(Max Conc. mg/mL);113.86(Max Conc. mM) - form A solid
- color White to off-white
- optical activity [α]/D -12.0 to -8.0°, c =1.0 in H2O
- Water Solubility H2O: 2mg/mL, clear
- InChIKey XPZQVLUPTGTIJP-NIBQMMLDNA-N
- FDA UNII X8T9SBH9LL
- UNSPSC Code 12352200
- NACRES NA.77
Safety
-
Symbol(GHS)


- Signal wordWarning
- Hazard statements H315-H319-H228
- Precautionary statements P240-P210-P241-P264-P280-P302+P352-P370+P378-P337+P313-P305+P351+P338-P362+P364-P332+P313
Diquafosol Tetrasodium Chemical Properties,Usage,Production
- Description Diquafosol (INS-365) was approved in Japan in 2010 as a 3% ophthalmic solution for treatment of dry eye disease. Clinical diagnosis of dry eye is difficult because the condition presents a variety of symptoms. Treatment options include tear supplements (lubricants), anti-inflammatory drugs (e.g., cyclosporine eye drops or steroid eye drops), and tear retention devices. Diquafosol is a unique agent for the treatment of dry eye in that it acts as a P2Y2 purinergic receptor agonist with the ability to activate this receptor on the ocular surface and stimulate water, lipid, and mucin secretion. Diquafosol is metabolized by phosphodiesterases to UTP, UDP, UMP, and uridine. Diquafosol has been well tolerated in clinical trials, with side effects being local to the ocular surface.In addition,no serious ocular or systemic adverse drug reactions were found during the clinical trials.
- Originator Inspire Pharmaceuticals (United States)
- Uses Enhancement of mucosal hydration in the treatment of chronic dry eye (P2Y2 receptor antagonist).
- brand name Diquas
- Clinical Use Diquafosol tetrasodium was approved in April 2010 as Diquas ® ophthalmic solution 3% for the treatment of dry eye syndrome and launched in Japan by Santen Pharmaceuticals. Diquafosol tetrasodium was originally discovered by Inspire Pharmaceuticals. In 2001, it was licensed to Santen for co-development and commercialization in Asian countries, and co-developed in collaboration with Allergan for the countries outside of Asia. In the U.S., diquafosol tetrasodium was submitted for a New Drug Application (NDA) as Prolacria ®(2% ophthalmic formulation) in June 2003. However, it is still in Phase III clinical development for dry eye syndrome. Diquafosol tetrasodium, also known as INS- 365, is a P2Y2 receptor agonist, which activates P2Y2 receptor on the ocular surface, leading to rehydration through activation of the fluid pump mechanism of the accessory lacrimal glands on the conjunctival surface.
-
Synthesis
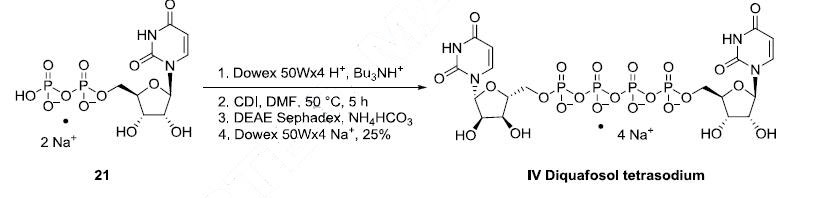
The large-scale synthesis route of diquafosol tetrasodium is described in
Scheme 4. Commercially available uridine 5?ˉ-diphosphate disodium salt (21) was transformed
into the corresponding tributylamine salt by ion exchange chromatography on Dowex 50 using Bu3NH+
phase, and then dimerized by means of CDI in DMF at 50 oC. The crude product was purified by
Sephadex DEAE column followed by ion exchange using a Dowex 50W resin in Na+ mode. The onepot
process provided diquafosol tetrasodium (IV) in 25% yield.
Diquafosol Tetrasodium Preparation Products And Raw materials
Raw materials
Preparation Products
Global(173)Suppliers
-
Supplier:
Guangzhou Tosun Pharmaceutical Limited
- Tel: +8618922120635
- Email:sales@toref-standards.com
- Country:China
- ProdList:998
- Advantage:58
-
Supplier:
Guangzhou Tosun Pharmaceutical Ltd
- Tel:+86-020-61855200-902<br/>+8618124244216
- Email:info@upharm.cn
- Country:China
- ProdList:897
- Advantage:58
-
Supplier:
Henan Tianfu Chemical Co.,Ltd.
- Tel:+86-0371-55170693<br/>+86-19937530512
- Email:info@tianfuchem.com
- Country:China
- ProdList:21628
- Advantage:55
-
Supplier:
ATK CHEMICAL COMPANY LIMITED
- Tel:+undefined-21-51877795
- Email:ivan@atkchemical.com
- Country:China
- ProdList:33024
- Advantage:60
-
Supplier:
Jinan Chenghui-Shuangda Chemical Co.,Ltd
- Tel:+86-531-58897082
- Email:ericqiao@jnchsd.com
- Country:CHINA
- ProdList:158
- Advantage:58
-
Supplier:
Standardpharm Co. Ltd.
- Tel:86-714-3992388
- Email:overseasales1@yongstandards.com
- Country:United States
- ProdList:14332
- Advantage:58
-
Supplier:
BOC Sciences
- Tel:+1-631-485-4226
- Email:inquiry@bocsci.com
- Country:United States
- ProdList:19552
- Advantage:58
-
Supplier:
TopScience Biochemical
- Tel:00852-68527855
- Email:info@itopbiochem.com
- Country:China Hong Kong
- ProdList:902
- Advantage:58
-
Supplier:
career henan chemical co
- Tel:+86-0371-86658258<br/>+8613203830695
- Email:factory@coreychem.com
- Country:China
- ProdList:29808
- Advantage:58
-
Supplier:
Hubei Ipure Biology Co., Ltd
- Tel: +8613367258412
- Email:ada@ipurechemical.com
- Country:China
- ProdList:10244
- Advantage:58
Related articles
211427-08-6, Diquafosol TetrasodiumRelated Search:
- 杂质
- 高纯试剂
- 杂质对照品
- 抑制剂
- 药靶配体
- 主打产品
- 二乙二醇甲乙醚
- 医用原料
- 有机化学
- 日用化学品
- 生物化工
- 试剂
- 医药原料
- 化学试剂
- 原料药
- 对照品-杂质对照品
- C18H26N4O23P44Na
- 敌敌畏四钠盐
- 地夸磷索四钠CRS
- 地夸磷索杂质11(地夸鳞索钠对照品)
- 地夸磷索U2P4
- 地夸磷索钠,地夸磷索四钠
- 地夸磷索杂质U2P4
- 地夸磷索钠对照品 211427-08-6
- 地夸磷索钠杂质
- P1,P4-二(尿苷5'-)四磷酸四钠
- 二喹福索钠
- 地夸磷索四钠,地夸磷索钠
- 地夸磷索四钠杂质
- 地夸磷索四钠中间体
- 地夸磷索杂质11
- 地夸磷索四钠盐
- 地夸磷索四钠
- 211427-08-6
- Uridine 5′-(pentahydrogen tetraphosphate), P′′′→5′-ester with uridine, sodium salt (1:4)
- CID 9876095
- Diquafosoftetrasodium
- Diquafosol Tetrasodium CRS
- Dexamethoxol tetrasodium
- TIANFUCHEM--211427-08-6 INS 365
- Diquafosol Sodium Reference Substance
- Diquafosol Tetrasodium (INS365)
- Diquafosol tetrasodium impurity
- P1,P4-Diuridine 5'-tetraphosphate tetrasodium salt
- Diquafosol tetrasodium intermediate
- Diquafosol Tetrasodium Salt
- Diquafosol Tetrasoudium or P1,P4-Di(Uridine-5′-Tetraphosphate)
- [[(2R,3S,4R,5R)-5-(2,4-dioxopyrimidin-1-yl)-3,4-dihydroxyoxolan-2-yl]methoxy-oxidophosphoryl] [[[(2R,3S,4R,5R)-5-(2,4-dioxopyrimidin-1-yl)-3,4-dihydroxyoxolan-2-yl]methoxy-oxidophosphoryl]oxy-oxidophosphoryl] phosphate
- Diquafosol Impurity 7(UP4U)
- Diquafosol tetrasodium (usan)
- Diquafosol tetrasodium
- Diquafosol sodium (jan)
- Diquafosol sodium
- D03864
- INS 365