ChemicalBook > CAS DataBase List > Ramelteon
Ramelteon
Ramelteon
- CAS No.196597-26-9
- Chemical Name:Ramelteon
- CBNumber:CB0496858
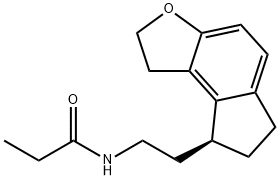
- Molecular Formula:C16H21NO2
- Formula Weight:259.34
- MOL File:196597-26-9.mol
Ramelteon Property
- Melting point 113-1150C
- alpha D20 -57.8° (c = 1.004 in chloroform)
- Boiling point 455.3±24.0 °C(Predicted)
- Density 1.119±0.06 g/cm3(Predicted)
- Flash point 2℃
- storage temp. Sealed in dry,Store in freezer, under -20°C
- solubility Dimethyl Sulfoxide, Ethanol, Methanol,
- form Solid
- pka 16.37±0.46(Predicted)
- color Crystalline
- optical activity [α]/D -50 to -60°, c =1.0 in chloroform-d
- InChI InChI=1S/C16H21NO2/c1-2-15(18)17-9-7-12-4-3-11-5-6-14-13(16(11)12)8-10-19-14/h5-6,12H,2-4,7-10H2,1H3,(H,17,18)/t12-/m0/s1
- InChIKey YLXDSYKOBKBWJQ-LBPRGKRZSA-N
- SMILES C(NCC[C@H]1C2=C3CCOC3=CC=C2CC1)(=O)CC
- CAS DataBase Reference 196597-26-9(CAS DataBase Reference)
- FDA UNII 901AS54I69
- NCI Drug Dictionary ramelteon
- ATC code N05CH02
- UNSPSC Code 12352200
- NACRES NA.77
Safety
- Hazard Codes :F,Xn
- Risk Statements :11-20/21/22-36
- Safety Statements :16-36/37
- RIDADR :UN 1648 3 / PGII
- WGK Germany :2
- HS Code :2932.99.7000
- Hazardous Substances Data :196597-26-9(Hazardous Substances Data)
-
NFPA 704:
0 2 0
-
Symbol(GHS)

- Signal wordWarning
- Hazard statements H302
- Precautionary statements P301+P312+P330
Ramelteon Price
More Price(5)
- Brand: Sigma-Aldrich(India)
- Product number: SML2262
- Product name : Ramelteon
- Purity:
- Packaging: 10MG
- Price: ₹13390.53
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: SML2262
- Product name : Ramelteon
- Purity:
- Packaging: 50MG
- Price: ₹53854.38
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: R-016
- Product name : Ramelteon solution
- Purity: 1.0?mg/mL in acetonitrile, ampule of 1?mL, certified reference material, Cerilliant?
- Packaging: 1ML
- Price: ₹22370.6
- Updated: 2022/06/14
- Buy: Buy
- Brand: TCI Chemicals (India)
- Product number: R0216
- Product name : Ramelteon
- Purity: min. 98.0 %
- Packaging: 100MG
- Price: ₹5100
- Updated: 2022/05/26
- Buy: Buy
- Brand: TCI Chemicals (India)
- Product number: R0216
- Product name : Ramelteon
- Purity: min. 98.0 %
- Packaging: 1G
- Price: ₹15000
- Updated: 2022/05/26
- Buy: Buy
Ramelteon Chemical Properties,Usage,Production
- Description Ramelteon, also known as N-[2-[(8S)-1,6,7,8-Tetrahydro-2H-indeno[5,4-b]furan-8-yl]ethyl]propanamide, is a melatonin agonist developed by Takeda Pharmaceuticals, Inc. It was approved by the FDA for marketing in the United States in September 2005 and is marketed under the name Rozerem. It is used to treat difficult-to-sleep and short-term insomnia. Ramelteon is effective for both chronic and short-term insomnia. Unlike most treatments of insomnia that target the GABA (g-aminobutyric acid) receptor complex, ramelteon is an agonist of the melatonin receptor. In particular, it has high selectivity for the MT1 and MT2 subtypes, which have been implicated in the maintenance of circadian rhythms, over the MT3 receptor responsible for other melatonin functions. Its lack of affinity for not only the GABA receptor complex but also neurotransmitter, dopaminerigic, opiate, and benzodiazepine receptors suggests an improved safety profile devoid of the abuse potential of the hypnotic drugs that target these receptors. As such, ramelteon is not a scheduled drug.
- Chemical Properties Crystalline Solid
- Originator Takeda (Japan)
- Uses Ramelteon is a selective melatonin receptor agonist of MT1 and MT2 approved for the treatment of insomnia (trouble in sleeping). It acts as a sedative and hypnotic agent. Ramelteon is the only prescription sleep aid not designated as a Schedule IV controlled substance.
- Definition ChEBI: N-[2-[(8S)-2,6,7,8-tetrahydro-1H-cyclopenta[e]benzofuran-8-yl]ethyl]propanamide is a member of indanes.
- brand name Rozerem (Takeda).
- General Description The melatonin molecule was modified mainly by replacing the nitrogen of the indole ring with a carbon to give an indane ring and by incorporating 5-methoxyl group in the indole ring into a more rigid furan ring. The selectivity of the resulting ramelteon for MT1 receptor is eight times more than that of MT2 receptor. Unlike melatonin, it is more effective in initiating sleep (MT1 activity) rather than to readjust the circadian rhythm (MT2 activity). It appears to be distinctly more efficacious than melatonin but less efficacious than benzodiazepines as a hypnotic. Importantly, this drug has no addiction liability (it is not a controlled substance). As a result, it has recently been approved for the treatment of insomnia.
-
Synthesis
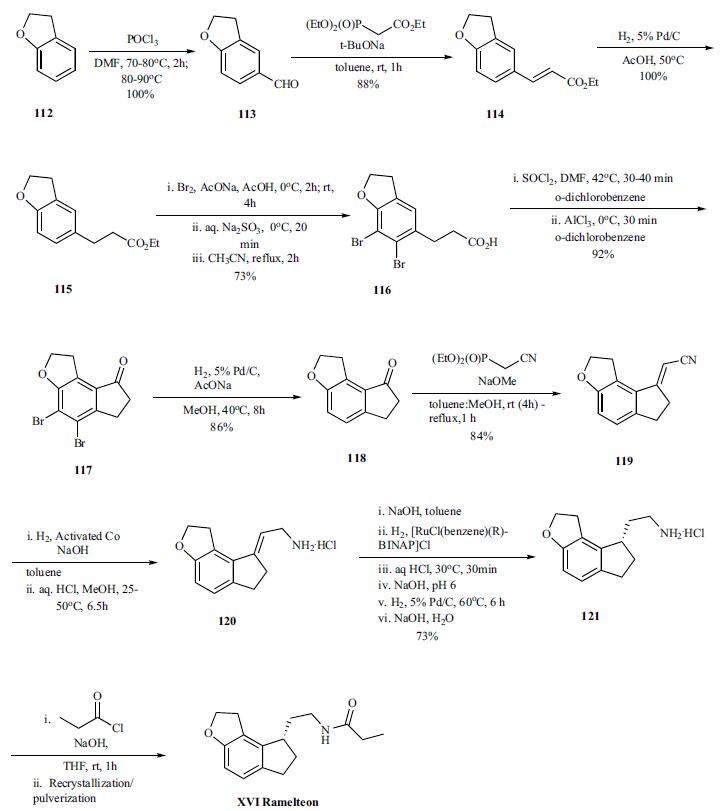
Vilsmeier-Haack reaction on benzofuran 112 provided aldehyde 113 (100%), which was converted to olefin 114 (88%) by Horner-Emmons reaction with triethylphosphonoacetate, and was followed by hydrogenation of the olefin to give ester 115 (100%). In order to avoid the cyclization of the acid chloride intermediate into the wrong position, the benzene ring was protected by bromination. Both bromination and hydrolysis of the ester is accomplished in a single pot to give acid 116. Thus the ester is brominated with bromine in sodium acetate and acetic acid at 0°C and RT for several hours followed by quenching of remaining bromide by sodium thiosulfate. The resulting acidic solution was taken up in acetonitrile and refluxed for 2hr to provide the acid 116 in 73% yield. The conversion of the acid to acid chloride was done by reacting with thionyl chloride in odichlorobenzene at 40°C for 30 to 40 min after which the reaction was cooled to 0°C . Aluminum trichloride was added and the reaction mixture was stirred at 0°C for 30 min to deliver cyclized ketone 117 in 92% yield. After completion of the cyclization, the bromines are removed by hydrogenation (86%) and resulting ketone 118 was then reacted under Horner-Emmons condition with diethyl cyano phosphonate to give vinyl nitrile 119 in 84% yield. Selective reduction of the nitrile was accomplished by hydrogenation under basic condition (sodium hydroxide in toluene) in the presence of the activated cobalt at 25-50°C for 6.5 hr. The amine was recovered as hydrochloride salt 120 (99% yield) by treating the amine with HCl in methanol. In the next step, the amine salt 120 was taken up in toluene and treated with sodium hydroxide followed by hydrogenation of the mixture with [RuCl(benzene)(R)-BINAP]Cl as catalyst to provide chiral amine 121, after several work up and palladium catalyzed hydrogenations, in 73% overall yield. Final acylation of the amine with propionyl chloride in the presence of aqueous sodium hydroxide in THF at room temperature gave the desired product ramelteon (XVI), after crystallization, in 97% yield.
- Drug interactions Since CYP1A2 is the major isozyme involved in the hepatic metabolism of ramelteon, it should not be taken in combination with strong CYP1A2 inhibitors, such as fluvoxamine. Co-administration with either ketoconazole (a CYP3A4 inhibitor) or fluconazole (a potent CYP2C9 inhibitor) resulted in significant increases in AUC and Cmax, but the extensive metabolism and highly variable plasma concentrations of ramelteon precluded the need for dose modification. The package insert, however, cautions patients about co-administration with potent CYP3A4 and CYP2C9 inhibitors.
- storage Store at -20°C
-
References
[1] KATOKOKI. Neurochemical properties of ramelteon (TAK-375), a selective MT1/MT2 receptor agonist.[J]. Neuropharmacology, 2005. DOI:10.1016/j.neuropharm.2004.09.007.
[2] MIYAMOTO M. Pharmacology of Ramelteon, a Selective MT1/MT2 Receptor Agonist: A Novel Therapeutic Drug for Sleep Disorders[J]. CNS Neuroscience & Therapeutics, 2009. DOI:10.1111/j.1755-5949.2008.00066.x.
[3] MCGECHANADAM WellingtonKeri. Ramelteon.[J]. CNS drugs, 2005. DOI:10.2165/00023210-200519120-00007.
[4] BORJANANCY L DanielKaren L. Ramelteon for the treatment of insomnia.[J]. Clinical therapeutics, 2006. DOI:10.1016/j.clinthera.2006.10.016.
Ramelteon Preparation Products And Raw materials
Raw materials
Preparation Products
Global(402)Suppliers
-
Supplier:
Henan Bao Enluo International TradeCo.,LTD
- Tel:+86-17331933971<br/>+86-17331933971
- Email:deasea125996@gmail.com
- Country:China
- ProdList:2472
- Advantage:58
-
Supplier:
Capot Chemical Co.,Ltd.
- Tel:+86-(0)57185586718<br/>+86-13336195806
- Email:sales@capot.com
- Country:China
- ProdList:29730
- Advantage:60
-
Supplier:
Beijing Cooperate Pharmaceutical Co.,Ltd
- Tel:010-60279497
- Email:sales01@cooperate-pharm.com
- Country:CHINA
- ProdList:1803
- Advantage:55
-
Supplier:
Henan Tianfu Chemical Co.,Ltd.
- Tel:+86-0371-55170693<br/>+86-19937530512
- Email:info@tianfuchem.com
- Country:China
- ProdList:21628
- Advantage:55
-
Supplier:
Shanghai Time Chemicals CO., Ltd.
- Tel:+86-021-57951555<br/>+8617317452075
- Email:jack.li@time-chemicals.com
- Country:China
- ProdList:1803
- Advantage:55
-
Supplier:
ATK CHEMICAL COMPANY LIMITED
- Tel:+undefined-21-51877795
- Email:ivan@atkchemical.com
- Country:China
- ProdList:33024
- Advantage:60
-
Supplier:
career henan chemical co
- Tel:+86-0371-86658258<br/>+8613203830695
- Email:sales@coreychem.com
- Country:China
- ProdList:29859
- Advantage:58
-
Supplier:
Shanghai Arbor Chemical Co., Ltd.
- Tel:021-60451682
- Email:act@arborchemical.com
- Country:CHINA
- ProdList:904
- Advantage:58
-
Supplier:
Shenzhen Nexconn Pharmatechs Ltd
- Tel:+86-755-89396905<br/>+86-15013857715
- Email:admin@nexconn.com
- Country:China
- ProdList:10406
- Advantage:58
-
Supplier:
Hubei Jusheng Technology Co.,Ltd.
- Tel:18871490254
- Email:linda@hubeijusheng.com
- Country:CHINA
- ProdList:28172
- Advantage:58
Related articles
196597-26-9, RamelteonRelated Search:
- Chiral Reagents
- Pharmaceuticals
- Intermediates & Fine Chemicals
- Other APIs
- ROZEREM
- 高纯试剂
- 科研原料
- 高纯化学试剂
- 抑制剂
- 配体家族
- 药靶配体
- 杂质对照品
- 标准品
- 化工
- 化学试剂
- 科研试剂
- 原料药API
- 医用原料
- 化工原料
- 镇静催眠类抑制剂
- 原料药
- G蛋白偶联受体&G蛋白
- 医药原料
- 小分子抑制剂
- 雷美替胺,10 MM DMSO 溶液
- 雷美替胺|||TAK-375
- 雷美顿
- (S)-N-(2-(2,6,7,8-四氢-1H-茚并[5,4-B]呋喃-8-基)乙基)丙酰胺
- 拉梅尔通
- 雷美替胺/瑞美替胺/
- 瑞美替昂
- 雷美替胺
- 雷美尔通
- 拉米替隆
- (S)-N-[2-(1,6,7,8-四氢-2H-茚并[5,4-b]呋喃-8-基)乙基]丙酰胺
- 瑞美替胺196597-26-9
- 雷美替胺 别名: RAMELTEON
- 瑞美替昂 -D5
- 瑞美替胺
- 196597-26-9
- Ramelteon, 10 mM in DMSO
- Rametylamine
- Ray for amine
- N-[2-[(8S)-1,6,7,8-Tetrahydro-2H-indeno[5,4-b]furan-8-yl]ethyl]propanamide
- (S)-N-(2-(2,6,7,8-tetrahydro-1H-indeno[5,4-b]furan-8-yl)ethyl)propionamide
- Rozerem
- Rhodialux
- P Sanduvor 3035
- RamelteonQ: What is Ramelteon Q: What is the CAS Number of Ramelteon Q: What is the storage condition of Ramelteon Q: What are the applications of Ramelteon
- DF883
- Ramelteon USP/EP/BP
- Propanamide, N-[2-[(8S)-1,6,7,8-tetrahydro-2H-indeno[5,4-b]furan-8-yl]ethyl]-
- UNII-901AS54I69
- N-[2-[(8S)-2,6,7,8-tetrahydro-1H-cyclopenta[e][1]benzofuran-8-yl]ethyl]propanamide
- TAK-375; ROZEREM; TAK375; TAK 375;
- CS-1962
- Ramelteon, >=99%
- Ramelteon solution