ChemicalBook > CAS DataBase List > gamma-Linolenic acid
gamma-Linolenic acid
gamma-Linolenic acid
- CAS No.506-26-3
- Chemical Name:gamma-Linolenic acid
- CBNumber:CB3238150
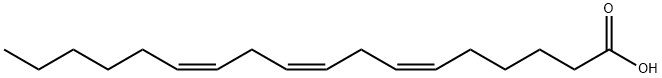
- Molecular Formula:C18H30O2
- Formula Weight:278.43
- MOL File:506-26-3.mol
gamma-Linolenic acid Property
- Melting point -12~-11℃
- Boiling point 379.5±11.0 °C(Predicted)
- Density 0.92
- refractive index n
20/D 1.471 - Flash point 110 °C
- storage temp. -20°C
- solubility Chloroform (Sparingly), DMSO (Slightly), Methanol (Slightly)
- form liquid
- pka 4.74±0.10(Predicted)
- color Colourless
- biological source synthetic
- Merck 14,5507
- BRN 1712253
- Stability Light Sensitive
- InChIKey VZCCETWTMQHEPK-QNEBEIHSSA-N
- LogP 6.570 (est)
- CAS DataBase Reference 506-26-3(CAS DataBase Reference)
- FDA UNII 78YC2MAX4O
- NIST Chemistry Reference Gamolenic acid(506-26-3)
- ATC code D11AX02,D11AX52
- UNSPSC Code 85151701
- NACRES NA.25
Safety
-
Symbol(GHS)

- Signal wordWarning
- Hazard statements H315-H319-H335
- Precautionary statements P261-P264-P271-P280-P302+P352-P305+P351+P338
gamma-Linolenic acid Price
More Price(8)
- Brand: Sigma-Aldrich(India)
- Product number: L2378
- Product name : γ-Linolenic acid
- Purity: ≥99%, liquid
- Packaging: 10MG
- Price: ₹6126.95
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: L2378
- Product name : γ-Linolenic acid
- Purity: ≥99%, liquid
- Packaging: 100MG
- Price: ₹14797.78
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: L2378
- Product name : γ-Linolenic acid
- Purity: ≥99%, liquid
- Packaging: 500MG
- Price: ₹42423.18
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: L2378
- Product name : γ-Linolenic acid
- Purity: ≥99%, liquid
- Packaging: 1G
- Price: ₹54070.88
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: 62174
- Product name : γ-Linolenic acid
- Purity: analytical standard
- Packaging: 100MG
- Price: ₹18878.8
- Updated: 2022/06/14
- Buy: Buy
gamma-Linolenic acid Chemical Properties,Usage,Production
- Description γ-Linolenic acid (gamma-linolenic acid or GLA, INN and USAN gamolenic acid) is a fatty acid found primarily in vegetable oils. It is sold as a dietary supplement for treating problems with inflammation and auto-immune diseases, although its efficacy is disputed.
-
Description
γ-
Linolenic acid (GLA) is an ω- 6 fatty acid which can be elongated to arachidonic acid for endogenous eicosanoid synthesis. It is a weak LTB4 receptor antagonist, inhibiting [3H]- LTB4 binding to porcine neutrophil membranes with a Ki of 1 μM. GLA produces 53% inhibition at a 1 mg/kg dose in an in vivo model of LTB4- induced bronchoconstriction. - Chemical Properties GLA is categorized as an n?6 (also called ω?6 or omega-6) fatty acid, meaning that the first double bond on the methyl end (designated with n or ω) is the sixth bond. In physiological literature, GLA is designated as 18:3 (n?6). GLA is a carboxylic acid with an 18-carbon chain and three cis double bonds. It is an isomer of α-linolenic acid, which is a polyunsaturated n?3 (omega-3) fatty acid, found in rapeseed canola oil, soy beans, walnuts, flax seed (linseed oil), perilla, chia, and hemp seed.
-
Occurrence
GLA is obtained from vegetable oils such as GLA safflower oil (Carthamus tinctorius), evening primrose (Oenothera biennis) oil, blackcurrant seed oil, borage oil, and hemp seed oil. GLA is also found in edible hemp seeds, oats, barley, and spirulina. Each contains varying amounts of the fatty acid, with GLA safflower oil at 40% GLA being a novel concentrated form. This is a new genetically modified oil and has been available in commercial quantities since 2011. It should be noted that conventional safflower oils have zero GLA. Borage oil ranges from 15 % to 20 % and evening primrose oil ranges from 8 % to 10 % GLA.
The human body produces GLA from linoleic acid (LA). This reaction is catalyzed by Δ6 - desaturase (D6D), an enzyme that allows the creation of a double bond on the sixth carbon counting from the carboxyl terminus. LA is consumed sufficiently in most diets, from such abundant sources as cooking oils and meats. However, a lack of GLA can occur when there is a reduction of the efficiency of the D6D conversion (for instance, as people grow older or when there are specific dietary deficiencies) or in disease states wherein there is excessive consumption of GLA metabolites. -
History
GLA was first isolated from the seed oil of evening primrose. This herbal plant was grown by Native Americans to treat swelling in the body. In the 17th century, it was introduced to Europe and became a popular folk remedy, earning the name king's cure-all. in 1919, Heiduschka and Lüft extracted the oil from evening primrose seeds and described an unusual linolenic acid, which they name γ-. Later, the exact chemical structure was characterized by Riley.
Although there are α- and γ- forms of linolenic acid, there is no β- form. One was once identified, but it turned out to be an artifact of the original analytical process. - Uses γ-linolenic acid has been used as an analytical standard in gas chromatography. It may be used in nutritional studies regarding weight regain and as a possible tumor suppression agent. γ-linolenic acid is used in studies on the mechanisms and prevention of oxidation/peroxidation of unsaturated fatty acids.
- Uses γ-Linolenic Acid is a fatty acid found mainly in vegetable oil. Studies suggest that γ-Linolenic Acid may have immune regulating and anti-inflammatory effects. γ-Linolenic Acid has also been used in the treatment of atopic eczeme although its efficacy is
- Definition ChEBI: A C18, omega-6 acid fatty acid comprising a linolenic acid having cis- double bonds at positions 6, 9 and 12.
- Biochem/physiol Actions Gamma-linolenate (C18:6,9,12) differs from α-linolenate (C18:9,12,15) in the positions of the double bonds.
-
References
[1] yagaloff k a, franco l, simko b, et al. essential fatty acids are antagonists of the leukotriene b4 receptor[j]. prostaglandins, leukotrienes and essential fatty acids, 1995, 52(5): 293-297.
[2] crooks s w, stockley r a. leukotriene b4[j]. the international journal of biochemistry & cell biology, 1998, 30(2): 173-178.
[3] tasset-cuevas i, fernández-bedmar z, lozano-baena m d, et al. protective effect of borage seed oil and gamma linolenic acid on dna: in vivo and in vitro studies[j]. plos one, 2013, 8(2): e56986.
[4] jamal g a, carmichael h. the effect of γ‐linolenic acid on human diabetic peripheral neuropathy: a double‐blind placebo‐controlled trial[j]. diabetic medicine, 1990, 7(4): 319-323.
[5] andreassi m, forleo p, dilohjo a, et al. efficacy of γ-linolenic acid in the treatment of patients with atopic dermatitis[j]. journal of international medical research, 1997, 25(5): 266-274.
gamma-Linolenic acid Preparation Products And Raw materials
Raw materials
Preparation Products
Global(276)Suppliers
-
Supplier:
Hebei Mujin Biotechnology Co.,Ltd
- Tel:+86 13288715578<br/>+8613288715578
- Email:sales@hbmojin.com
- Country:China
- ProdList:12810
- Advantage:58
-
Supplier:
BINBO BIOLOGICAL CO.,LTD
- Tel: +8618629063126
- Email:info@binbobiological.com
- Country:China
- ProdList:451
- Advantage:58
-
Supplier:
Henan Tianfu Chemical Co.,Ltd.
- Tel:+86-0371-55170693<br/>+86-19937530512
- Email:info@tianfuchem.com
- Country:China
- ProdList:21628
- Advantage:55
-
Supplier:
career henan chemical co
- Tel:+86-0371-86658258<br/>+8613203830695
- Email:sales@coreychem.com
- Country:China
- ProdList:29859
- Advantage:58
-
Supplier:
Chengdu Biopurify Phytochemicals Ltd.
- Tel:+86-28-82633860;<br/>+8618080483897
- Email:sales@biopurify.com
- Country:China
- ProdList:3772
- Advantage:58
-
Supplier:
Xiamen AmoyChem Co., Ltd
- Tel:+86-86-5926051114<br/>+8615060885618
- Email:sales@amoychem.com
- Country:China
- ProdList:6383
- Advantage:58
-
Supplier:
Hubei xin bonus chemical co. LTD
- Tel:86-13657291602
- Email:linda@hubeijusheng.com
- Country:CHINA
- ProdList:22963
- Advantage:58
-
Supplier:
Alchem Pharmtech,Inc.
- Tel:8485655694
- Email:sales@alchempharmtech.com
- Country:United States
- ProdList:63687
- Advantage:58
-
Supplier:
Masteam Bio-tech Co., Ltd.
- Tel:+86-15200345168<br/>+86-15200345168
- Email:bruce.lei@masteam.com
- Country:CHINA
- ProdList:151
- Advantage:58
-
Supplier:
TargetMol Chemicals Inc.
- Tel:+1-781-999-5354<br/>+1-00000000000
- Email:marketing@targetmol.com
- Country:United States
- ProdList:32159
- Advantage:58
Related articles
506-26-3, gamma-Linolenic acidRelated Search:
- Linolenic acid Docosahexaenoic Acid EPA Linoleic acid 6-Amino-4-hydroxy-2-naphthalenesulfonic acid Ethyl 2-(Chlorosulfonyl)acetate Arachidonic acid 4-Aminobutyric acid Folic acid Citric acid Ascoric Acid OLEIC ACID-1-13C OCTADECENEDIOIC ACID GAMMA-LINOLENIC ACID METHYL ESTER 1,2-OLEIN-3-LINOLENIN GAMMA-LINOLENIC ACID ETHYL ESTER HOMO-GAMMA-LINOLENIC ACID,DIHOMO-GAMMA-LINOLENIC ACID,(6Z,9Z,12Z)-octadeca-6,9,12-trienoic acid,DIHOMO-GAMMA-LINOLENIC ACID-CONTAINING TRIGLYCERIDE EVENING PRIMROSE OIL 10% GAMMA LINOLENIC ACID (GLA) OENOTHERA BIENNIS
- Unsaturated Higher Fatty Acids
- Higher Fatty Acids & Higher Alcohols
- Biochemistry
- Miscellaneous Natural Products
- Biological and chemical
- 抑制剂
- 代谢物
- 有机类
- 化工
- 有机产品
- 营养强化剂
- 精细化工品
- 化工原料
- 通用生化试剂-维生素
- 医药、农药及染料中间体
- 医药原料药
- 对照品-中药对照品
- 原料
- 有机化工原料
- 对照品
- 化学试剂
- 生化试剂-其他化学试剂
- 羧酸
- 食品添加剂
- 脂肪酸
- 有机砌块
- 通用试剂
- Higher Fatty Acids & Higher Alcohols
- Unsaturated Higher Fatty Acids
- Fatty Acids
- Chemopreventive Agents
- Cancer Research
- Anti-proliferative Agents
- Biochemicals and Reagents
- BioChemical
- Omega 6 fatty acids and derivatives
- Polyunsaturated
- Lipids
- Unsaturated fatty acids and derivatives
- Γ-亚麻酸,10 MM DMSO 溶液
- 十八碳三烯酸(顺-6,9,12)/Γ-亚麻酸
- 6-9-12十八碳三烯酸(Γ-亚麻酸)
- GAMOLENIC ACID|||Γ-LINOLENIC ACID|||Γ-亚麻酸
- (6Z,9Z,12Z)-十八碳-6,9,12-三烯酸
- 十八碳三烯酸(顺-6,9,12)(C18:3)
- Γ-亚麻酸(C18:3)
- (6Z,9Z,12Z)-十八烷基-6,9,12-三烯酸
- 异辛烷中Γ-亚麻酸溶液,1000UG/ML
- 十八碳三烯酸(顺-6,9,12)/Γ-亚麻酸(C18:3)标准品
- 全顺式-6
- 12-十八碳三烯酸
- 十八碳三烯酸(顺-6,9,12)
- 苯丙酮酸钠/SODIUM PHENYLPYRUVATE
- Γ-亚油酸
- Γ-亚麻酸油(GLA)
- R-亚麻酸/6,9,12十八碳三烯酸
- Γ-亚麻酸/6,9,12十八碳三烯酸
- R-亚麻酸粉