Uses in Cosmetics of gamma-Linolenic acid
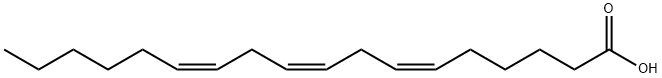
gamma-Linolenic acid or GLA (γ-linolenic acid) (INN: gamolenic acid) is a fatty acid found primarily in seed oils. When acting on GLA, arachidonate 5-lipoxygenase produces no leukotrienes and the conversion by the enzyme of arachidonic acid to leukotrienes is inhibited.GLA is categorized as an n−6 (also called ω−6 or omega-6) fatty acid, meaning that the first double bond on the methyl end (designated with n or ω) is the sixth bond. In physiological literature, GLA is designated as 18:3 (n−6). GLA is a carboxylic acid with an 18-carbon chain and three cis double bonds. It is an isomer of α-linolenic acid, which is a polyunsaturated n−3 (omega-3) fatty acid, found in rapeseed canola oil, soybeans, walnuts, flax seed (linseed oil), perilla, chia, and hemp seed.
GLA-Rich Vegetable Oils and Skin Health
GLA-rich vegetable oils have been evaluated in a variety of clinical conditions. The oils have been reported to have a skin barrier repairing effect, to normalize excessive transepidermal water loss (TEWL), and to improve mechanical and smoothness parameters after topical or systemic administration to healthy and irritated skin. In some skin diseases GLA-rich vegetable oils were found to have preventative or ameliorative effects. These observations suggest that GLA is important in the maintenance of skin health.
Sources of GLA
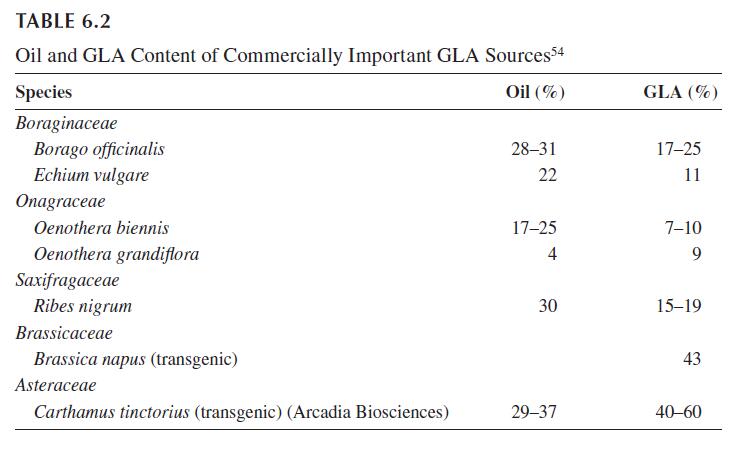
GLA is not common in fats and oils of vegetable or animal origin. Of the over 30 higher plant species in which the content of GLA in the oil exceeds 5%, only species from three genera are grown specifically as a source of GLA: Borago, Oenothera, and Echium. The seeds of blackcurrant (Ribes nigrum) are of minor commercial importance, they are a by-product of the fruit processing industry. Early attempts to develop GLA-rich yeasts and other fungal strains or cyanobacteria for fermentation appear to have been given up. A newer development is the high-level production of GLA in genetically modified seed crops, notably canola (Brassica napus) and safflower (Carthamus tinctoria). Table 6.2 lists the oil and GLA content of commercially important or potentially promising sources.
Mechanism of Action
The mechanism by which GLA exerts its skin health benefits is only partly understood. GLA has been reported to be particularly effective in conditions conducive toward local inflammation or where a disposition toward inflammation prevails.
Atopic eczema has a vigorous inflammatory component that contributes to redness and itching of the affected skin parts. Similarly, disruption of the permeability barrier in dry but healthy skin by chronic irritation of environmental and lifestyle stressors is invariably accompanied by an inflammation response. Experimentally, mechanical, chemical, or UV-induced stress has been shown to disrupt the cutaneous permeability barrier, to increase TEWL, to cause hyperproliferation of the skin, and to trigger the production of inflammatory epidermal cytokines such as TNF and IL-1. In winter, cold increases TEWL and can strip the skin of moisture, which combined with low humidity and indoor heating can cause dryness, and even cracking and chapping. Low humidity amplifies the hyperproliferative and inflammatory response to barrier disruption and may explain the seasonal exacerbation of dry skin and cutaneous disorders such as atopic eczema and psoriasis.
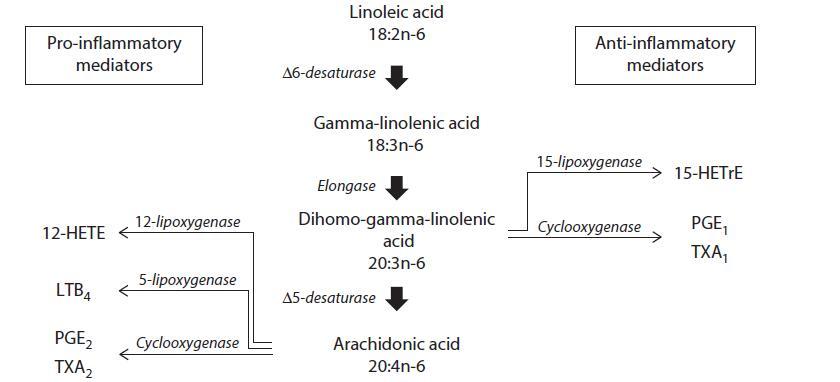
The prevailing theory behind the health benefits of GLA is that this fatty acid through its metabolite
DGLA mitigates inflammation. GLA is rapidly and efficiently transformed to DGLA by cyclooxygenase
to metabolites with potent anti-inflammatory activity such as prostaglandin E1 and 15-hydroxyeicosatrienoic
acid (15-HETrE). Furthermore, GLA suppresses, at least in vitro, the formation or
release of IL-1β and TNF-α, both important pro-inflammatory mediators. Alternatively, DGLA can be
metabolized to ARA, the precursor of eicosanoids that are mostly pro-inflammatory. Thus, DGLA is
the starting point of both a pro-inflammatory and an anti-inflammatory axis. There is evidence that in
humans and rodents the activity of 5D5 is slow and only fractions of DGLA are converted to ARA.41 At
least in inflammatory cells such as the neutrophil the majority of DGLA appears to be channeled towards
prostaglandin E1 and 15-HETrE formation. The increase in DGLA-derived eicosanoids relative to ARA
metabolites may represent a mechanism by which dietary GLA tips the balance toward a less aggressive
inflammatory response in acute and chronic cutaneous inflammations.
Irritation of skin—alone or on top of a restricted metabolism of LA to GLA—may not only disrupt
the skin barrier but may induce predisposed skin to inflammatory overreactions, i.e., the skin reacts
readily to minor insults with an overshooting or chronic inflammatory response. Similarly, the inflammatory
component of atopic eczema, psoriasis, and other skin affections might be in part the result of
inadequate amounts of prostaglandin E1 and 15-HETrE due to the lack of the substrate GLA5. PGE1
is notoriously low in atopic eczema sufferers. The shortage of PGE1 promotes the release of histamine
and other mediators from inflammatory cells, such as leukocytes, mast cells, and basophilic granulocytes,
aggravates the allergy inflammation reaction. The vicious circle may probably be maintained, as
histamin itself is immunosuppressive and inhibits D6D. Furthermore, apart from its anti-allergic and
anti-inflammatory activity, PGE1 has a marked immunoregulatory role. Shortness of PGE1 results in
a functional weakness of T-cell function, characterized by poor T-cell maturation and differentiation,
which may explain vulnerability of atopic eczema patients for bacterial infections. Of particular importance
is the impaired regulation of B-lymphocytes, the cells that are at least partially responsible for
the often-observed excessive IgE-response and the IgE concentrations function in the serum. However,
abnormal omega-6 PUFA metabolism is neither consistently found nor is it specifically associated with
atopic eczema but rather may be associated with IgE production and atopy in general.
If low GLA levels predispose skin to overshooting inflammation, oral or topical substitution of GLA could compensate epidermal deficits in this fatty acid. Strictly speaking, GLA is not a dietary essential fatty acid as humans can synthesize the molecule from LA via D6D. However, although LA deficiency is virtually non-existent in industrialized societies, the skin may still be undersupplied with GLA. The conversion of LA to GLA by D6D is generally a slow step. Furthermore, D6D is a very susceptible enzyme whose activity can be impaired genetically, by age, as well as by several nutritional deficiencies and lifestyle habits. Such physiological and pathological conditions that interfere with the bioconversion of LA lower body pools of GLA and its metabolites. Thus, the body, even in the presence of adequate dietary LA, may produce insufficient amounts of GLA. The skin is particularly sensitive to suboptimal GLA supply as it lacks the necessary D6D enzyme with which to form it in situ. Thus, the formation of skin GLA from LA must take place in other body tissues, notably the liver, from which the fatty acid is transported to the skin. Clearly, the skin is an organ that seems to depend on the supply of preformed GLA.
Kawashima et al. provided evidence that GLA acts through its metabolite DGLA. Oral administration of DGLA markedly suppressed atopic eczema-like skin lesions in a mouse model for atopic eczema. Clinical skin severity scores were significantly and dose-dependently lower in mice fed DGLA compared to control animals. Scratching behavior and plasma total IgE levels were also reduced in the DGLA group, in parallel with histological improvement. DGLA levels in the skin, spleen, and plasma were increased. Discontinuation of DGLA administration led to a flare-up of dermatitis and a decrease in DGLA contents in the skin, spleen, and plasma. These findings indicate that oral administration of DGLA effectively prevents the development of atopic eczema in this mouse model, and that DGLA in phospholipids is possibly a compound of key importance in the development and prevention of dermatitis.
Further evidence for the pivotal role of D6D in the maintenance of a healthy skin was the finding that D6D knockout mice develop severe ulcerative dermatitis despite abundant LA in tissues. Skin prostaglandin D2, a modulator of skin reaction to irritants, was decreased to 10% of the wild type controls.
Conclusions
Patients at risk of or with a chronic skin disease with an inflammatory component such as atopic eczema, psoriasis, or seborrheic dermatitis could benefit from topical or systemic adjuvant treatment with GLArich vegetable oils. Similarly, in stressed healthy skin, EPO and BO have a skin barrier repairing effect, normalize excessive TEWL, and improve several biophysical skin parameters. The effects appear to be mediated through anti-inflammatory metabolites of GLA.
REFERENCES
11. Ziboh VA, Chapkin RS. Biologic significance of polyunsaturated fatty acids in the skin. Arch Dermatol
1987;123:1686a–90.
12. Chung S, Kong S, Seong K et al. Gamma-linolenic acid in borage oil reverses epidermal hyperproliferation
in guinea pigs. J Nutr 2002;132:3090–7.
13. Fan YY, Chapkin RS. Mouse peritoneal macrophage prostaglandin E1 synthesis is altered by dietary
gamma-linolenic acid. J Nutr 1992;122:1600–6.
14. Lawson LD, Hughes BG. Triacylglycerol structure of plant and fungal oils containing γ-linolenic acid.
Lipids 1988;23:313–7.
15. Farage MA, Maibach HI. Sensitive skin: Closing in on a physiological cause. Contact Dermatitis
2010;62:137–49.
16. Nissen HP, Biltz H, Muggli R. Borage oil. Gamma-linolenic acid decreases skin roughness and TEWL
and increases skin moisture in normal and irritated human skin. Cosmetics Toiletries 1995;110:71–4.
17. Morse NL, Clough PM. A meta-analysis of randomized, placebo-controlled clinical trials of Efamol evening
primrose oil in atopic eczema. Where do we go from here in light of more recent discoveries? Curr
Pharm Biotechnol 2006;7:503–24.
18. Bamford JT, Ray S, Musekiwa A et al. Oral evening primrose oil and borage oil for eczema. Cochrane
Database Syst Rev 2013;4:CD004416.
19. Yen CH, Dai YS, Yang YH et al. Linoleic acid metabolite levels and transepidermal water loss in children
with atopic dermatitis. Ann Allergy Asthma Immunol 2008;100:66–73.
20. Oliwiecki S, Burton JL, Elles K et al. Levels of essential and other fatty acids in plasma and red cell phospholipids
from normal controls and patients with atopic eczema. Acta Derm Venereol 1991;71:224–8.
You may like
Related articles And Qustion
See also
Lastest Price from gamma-Linolenic acid manufacturers

US $0.00/kg2025-04-02
- CAS:
- 506-26-3
- Min. Order:
- 1kg
- Purity:
- 10% 12% 20%
- Supply Ability:
- 3000 kg