ChemicalBook > CAS DataBase List > SWAINSONINE
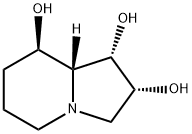
SWAINSONINE
SWAINSONINE
- CAS No.72741-87-8
- Chemical Name:SWAINSONINE
- CBNumber:CB0484750
- Molecular Formula:C8H15NO3
- Formula Weight:173.21
- MOL File:72741-87-8.mol
SWAINSONINE Property
- Melting point 148-149°C
- Boiling point 353.3±21.0 °C(Predicted)
- Density 1.38±0.1 g/cm3 (20 ºC 760 Torr)
- storage temp. -20°C
- solubility H2O: soluble1mg/mL
- pka 14.01±0.60(Predicted)
- form lyophilized powder
- color white to faint yellow
- biological source synthetic
- Water Solubility Soluble to 50 mM in water
- BRN 4175740
- Stability Stable for 1 year from date of purchase as supplied. Solutions in DMSO, ethanol or distilled water may be stored at -20°C for up to 3 months.
- CAS DataBase Reference 72741-87-8
- FDA UNII RSY4RK37KQ
- UNSPSC Code 51102829
- NACRES NA.85
Safety
-
Symbol(GHS)

- Signal wordWarning
- Hazard statements H302-H312-H332
- Precautionary statements P280
SWAINSONINE Price
More Price(3)
- Brand: Sigma-Aldrich(India)
- Product number: S9263
- Product name : Swainsonine
- Purity: synthetic
- Packaging: 0.1MG
- Price: ₹17341.65
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: S9263
- Product name : Swainsonine
- Purity: synthetic
- Packaging: 0.5MG
- Price: ₹50412.03
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: S8195
- Product name : Swainsonine
- Purity: from Metarrhizium anisopliae, ≥98% (TLC)
- Packaging: 1MG
- Price: ₹24388.73
- Updated: 2022/06/14
- Buy: Buy
SWAINSONINE Chemical Properties,Usage,Production
- Description Swainsonine (72741-87-8) is a naturally occurring alkaloidal toxin found in locoweed. Inhibits the biosynthesis of complex glycoproteins by inhibition of Golgi mannosidase II (IC50 = 0.2 mM).1 Inhibits growth and potentiates the cytotoxic effect of taxol in hepatocellular carcinoma in vivo.2 Induces apoptosis in a variety of cell types including cerebral cortical neurons.3 Impairs adult neurogenesis and spatial learning and memory.4? Abrogation of complex glycosylation by swainsonine results in strain-and cell-specific inhibition of prion replication.5 Induces lysosomal storage disease in farm animals.6
- Chemical Properties White Crystalline Solid
- Occurrence Swainsonia canescens yields this simple alkaloid.
- Uses Swainsonine is an indolizidine alkaloid naturally found in certain plants including locoweed that inhibits N-linked glycoside hydrolases, preventing the processing of asparagine-linked glycoproteins. It reversibly inhibits lysosomal α-mannosidase and Golgi α-mannosidase II (IC50 = 0.2 μM). Swainsonine is used to study the role of N-linked glycosylation in cellular processes and has been shown to have antiproliferative and antimetastatic effects of cancer cells in culture and in mice. The inhibition of α-mannosidase activity in lysosomes produces an accumulation of partially-processed oligosaccharides and glycoproteins, giving rise to lysosomal storage disease. Swainsonine toxicity in herbivores results in a condition known as locoism, characterized by hyperactivity, aggression, stiff and clumsy gait, low head carriage, salivation, seizures, and apparent blindness, culminating in increased miscoordination, weakness and death.
- Uses Swainsonine is a plant alkaloid derived from Swainsona canescens (a leguminous plant). It is a reversible, active-site directed inhibitor of a-mannosidase at concentrations of 5-10mM. At acid pH, swainsonine resembles an intermediate in the hydrolysis of mannosidases.Swainsonine completely inhibits mammalian Golgi a-mannosidase II (a-3/6-mannosidase in the glycoprotein processing pathway) and mammalian lysosomal a-mannosidase (acid mannosidase). At higher concentrations, swainsonine also inhibits mammalian cytosolic a-mannosidase. It has been shown to inhibit growth of transformed fibroblasts in soft agar and to enhance the antiproliferative effects of INF on murine lymphoreticular tumor cells in vitro. It also blocks the expression of b1-6 branched complex-type oligosaccharides and shun
- Definition ChEBI: An indolizidine alkaloid isolated from the plant Swainsona canescens with three hydroxy substituents at positions 1, 2 and 8.
- General Description Swainsonine is produced by endophytes, plant and insect pathogens. It is synthesized from the pipecolic acid and mevalonic acid. Swainsonine is a water-soluble indole alkaloid.
- Biological Activity Inhibitor of α -mannosidase II which inhibits glycoprotein processing. Displays anticancer and immune modulatory properties.
- Biochem/physiol Actions Swainsonine is a potent α-mannosidase inhibitor. It also has antimetastatic, antiproliferative, and immunomodulatory activity . It also inhibits glycoprotein processing.
- storage Store at -20°C
- References 1) Tulsiani et al. (1985), Marked differences in the swainsonine inhibition of rat liver lysomal alpha-D-mannosidase, rat liver Golgi mannosidase II, and jack bean alpha-D-mannosidase; Arch. Biochem. Biophys., 236 427 2) You et al. (2012), Swainsonine inhibits growth and potentiates the cytotoxic effect of paclitaxel in hepatocellular carcinoma in vitro and in vivo; Oncol. Rep., 28 2091 3) Lu et al. (2015), Swainsonine-induced apoptosis pathway in cerebral cortical neurons; Res. Vet. Sci., 102 34 4) Wang et al. (2015), Exposure to swainsonine impairs adult neurogenesis and spatial learning and memory; Toxicol. Lett., 232 263 5) Browning et al. (2011), Abrogation of complex glycosylation by swainsonine results in strain- and cell-specific inhibition of prion replication; J. Biol. Chem., 286 40962 6) Dantas et al. (2007), Swainsonine-induced lysosomal storage disease in goats caused by the ingestion of Turbina cordata in Northeastern Brazil; Toxicon, 49 111
SWAINSONINE Preparation Products And Raw materials
Raw materials
Preparation Products
Global(182)Suppliers
-
Supplier:
Shanghai Zheyan Biotech Co., Ltd.
- Tel:18017610038
- Email:zheyansh@163.com
- Country:CHINA
- ProdList:3619
- Advantage:58
-
Supplier:
Chengdu Biopurify Phytochemicals Ltd.
- Tel:+86-28-82633860;<br/>+8618080483897
- Email:sales@biopurify.com
- Country:China
- ProdList:3772
- Advantage:58
-
Supplier:
Hubei Jusheng Technology Co.,Ltd.
- Tel:18871490254
- Email:linda@hubeijusheng.com
- Country:CHINA
- ProdList:28172
- Advantage:58
-
Supplier:
Hubei xin bonus chemical co. LTD
- Tel:86-13657291602
- Email:linda@hubeijusheng.com
- Country:CHINA
- ProdList:22963
- Advantage:58
-
Supplier:
BOC Sciences
- Tel:+1-631-485-4226
- Email:inquiry@bocsci.com
- Country:United States
- ProdList:19552
- Advantage:58
-
Supplier:
career henan chemical co
- Tel:+86-0371-86658258<br/>+8613203830695
- Email:factory@coreychem.com
- Country:China
- ProdList:29808
- Advantage:58
-
Supplier:
Wuhan ChemNorm Biotech Co.,Ltd.
- Tel:+86-27-8439 4403<br/>18971486879
- Email:sales@chemnorm.com
- Country:CHINA
- ProdList:2935
- Advantage:58
-
Supplier:
ANHUI WITOP BIOTECH CO., LTD
- Tel: +8615255079626
- Email:eric@witopchemical.com
- Country:China
- ProdList:23541
- Advantage:58
-
Supplier:
Shandong Zhishang New Material Co., Ltd.
- Tel: +8617653113209
- Email:sales002@sdzschem.com
- Country:China
- ProdList:3049
- Advantage:58
-
Supplier:
Hefei TNJ Chemical Industry Co.,Ltd.
- Tel:+86-0551-65418684<br/>+8618949823763
- Email:sales@tnjchem.com
- Country:China
- ProdList:25356
- Advantage:58
72741-87-8, SWAINSONINERelated Search:
- CONDURITOL B EPOXIDE (-)-Gossypol Mitomycin C C2 CERAMIDE 1-Hexanol Betaine 1-Octacosanol SWAINSONINE(P) 1,2-di-epi-swainsonine swainsonine N-oxide L-(+)-SWAINSONINE 1,2-ISOPROPYLIDENE SWAINSONINE SWAINSONINE-6,7-3H 6,7-DIHYDROXYSWAINSONINE SWAINSONINE (1S,2R,8R,8AR)-1,2,8-TRIACETOXY-OCTAHYDRO-5-OXYINDOLIZINE
- Elisa Kit-plant ELISA Kit
- Inhibitors
- Glycosidase Inhibitors
- Alkaloids
- 中药标准品,对照品
- 生化试剂
- 小分子
- 标准品 -中药标准品
- 化工原料
- 对照品-中药对照品
- 分析试剂标准品
- 碳水化合物
- 植物提取物
- 标准品
- Antibiotics A to Z
- Antibiotics N-S
- Antibiotics
- Biochemicals and Reagents
- BioChemical
- Substrate Analogs
- Mannosidase, alpha-
- L to O
- Enzyme Inhibitors
- Enzyme Inhibitors by Enzyme
- Enzyme Inhibitors by Type
- Enzymes, Inhibitors, and Substrates
- SWAINSONINE,LYSOSOMAL ALPHA-MANNOSIDASE抑制剂
- 植物苦马豆素ELISA试剂盒
- 史瓦宁
- 苦马豆素/八倾吲嗪三醇
- 苦马豆碱
- D-苦马豆素
- 苦马豆素八倾吲嗪三醇D-苦马豆素(1S
- 8-八氢吲哚啶三醇
- (1S,2R,8R,8AR)-1,2,8-八氢吲哚啶三醇
- 八倾吲嗪三醇
- 苦马豆素
- 72741-87-8
- Swainsonine, <i>Swainsona canescens</i>
- Swainsonine, lysosomal alpha-mannosidase inhibitor
- (-)-Swainsonine
- 1,2,8-Indolizinetriol,octahydro-, (1S,2R,8R,8aR)-
- Swainsonine, Swainsona canescens - CAS 72741-87-8 - Calbiochem
- SWAINSONINE
- SWAINSONINE FROM LOCOWEED, 500 UG*
- 8AR)-1
- Swainsonine min. 99%
- (1S,2R,8R,8aR)-1,2,3,5,6,7,8,8a-Octahydroindolizine-1,2,8-triol
- 8α,β-Octahydroindolizidine-1α,2α,8β-triol
- (-)-SWAINSONINE (1S,2R,8R,8AR)-1,2,8-OCTAHYDROINDOLIZIDINETRIOL (1S,2S,8R,8AR)-TRIHYDROXYINDOLIZIDINE
- 8-indolizinetriol,octahydro-,(1s-(1-alpha,2-alpha,8-beta,8a-beta))-2
- 8-indolizinetriol(1s-(1-alpha,2-alpha,8-beta,8a-beta))-octahydro-2
- SWAINSONINE, SWAINSONA CANESCENS
- 8a,-Octahydroindolizidine-1a,2a,8-triol
- SwainMoia
- (1S,2R,8R,8aR)-Octahydro-1,2,8-indolizinetriol
- D-Swainsonine
- (1S,8aβ)-Octahydroindolizine-1α,2α,8β-triol