ChemicalBook > CAS DataBase List > Venlafaxine hydrochloride
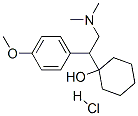
Venlafaxine hydrochloride
Venlafaxine hydrochloride
- CAS No.99300-78-4
- Chemical Name:Venlafaxine hydrochloride
- CBNumber:CB0136181
- Molecular Formula:C17H28ClNO2
- Formula Weight:313.86
- MOL File:99300-78-4.mol
Venlafaxine hydrochloride Property
- Melting point 207-209°C
- Flash point 9℃
- storage temp. 2-8°C
- solubility H2O: >10mg/mL
- form powder
- color white
- Water Solubility water: 100mM
DMSO: 50mM - Merck 14,9946
- BCS Class 1
- InChIKey QYRYFNHXARDNFZ-UHFFFAOYSA-N
- CAS DataBase Reference 99300-78-4(CAS DataBase Reference)
- NCI Dictionary of Cancer Terms Effexor
- FDA UNII 7D7RX5A8MO
- NCI Drug Dictionary Effexor
- EPA Substance Registry System Cyclohexanol, 1-[2-(dimethylamino)-1-(4-methoxyphenyl)ethyl]-, hydrochloride (1:1 (99300-78-4)
- UNSPSC Code 41116107
- NACRES NA.77
Safety
- Hazard Codes :Xi,T,F
- Risk Statements :36/37/38-36-39/23/24/25-23/24/25-11
- Safety Statements :26-37/39-45-36/37-16-7
- RIDADR :UN1230 - class 3 - PG 2 - Methanol, solution
- WGK Germany :3
- RTECS :GV8872760
- HS Code :29225090
-
NFPA 704:
0 2 0
-
Symbol(GHS)

- Signal wordDanger
- Hazard statements H411
- Precautionary statements P273-P391-P501
Venlafaxine hydrochloride Price
More Price(6)
- Brand: Sigma-Aldrich(India)
- Product number: V7264
- Product name : Venlafaxine hydrochloride
- Purity: ≥98% (HPLC), powder
- Packaging: 10MG
- Price: ₹15598.83
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: V7264
- Product name : Venlafaxine hydrochloride
- Purity: ≥98% (HPLC), powder
- Packaging: 50MG
- Price: ₹73350.2
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: V-004
- Product name : Venlafaxine hydrochloride solution
- Purity: 1.0?mg/mL in methanol (as free base), ampule of 1?mL, certified reference material, Cerilliant?
- Packaging: 1ML
- Price: ₹8751.4
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: PHR1736
- Product name : Venlafaxine Hydrochloride
- Purity: Pharmaceutical Secondary Standard; Certified Reference Material
- Packaging: 1G
- Price: ₹11420.38
- Updated: 2022/06/14
- Buy: Buy
- Brand: TCI Chemicals (India)
- Product number: V0110
- Product name : Venlafaxine Hydrochloride
- Purity:
- Packaging: 1G
- Price: ₹6100
- Updated: 2022/05/26
- Buy: Buy
Venlafaxine hydrochloride Chemical Properties,Usage,Production
- Synthesis Venlafaxine (92.4 g) was dissolved in ethyl acetate (450 ml) at 50-55 ºC, filtered hot then cooled to 10-15 ºC and pH was adjusted to <2 with addition of isopropyl alcohol saturated with HCl gas and allowed to stand for 15 minutes. The white solid was filtered off, washed with EtOAc (75 ml), and then with petroleum ether (150 ml). The crystalline salt was dissolved in MeOH (160 ml) at 50-55 ºC and filtered when hot. The hydrochloride salt 35 was precipitated by adding ethyl acetate drop-wise, at room temperature. After 4 hours the solid was filtered and washed with ethyl acetate (100 ml). The solid (98.3 g) was allowed to dry in air (6-8 hours).
- Description Venlafaxine hydrochloride, a novel phenethylamine derivative, was introduced in the U.S.A. as an antidepressant. Venlafaxine is reported to be the first in the class of the second-generation of antidepressants with dual serotonidnorepinephrine reuptake inhibitory activity. Venlafaxine lacks any affinity for muscarinic, cholinergic, histaminergic and noradrenergic receptors and therefore, has an unusually favorable side effect profile compared with classical tricyclic antidepressants and shows less cardiotoxicity. In addition, venlafaxine has a rapid onset of action that makes it unique among the antidepressant agents. Other indications for venlafaxine include the treatment of obsessive and panic disorders, and obesity. Both enantiomers of venlafaxine were reported to have similar biological activity.
- Chemical Properties White Crystalline Powder
- Originator Wyeth-Ayerst (U.S.A.)
- Uses A selective serotonin noradrenaline reuptake inhibitor. Used as an antidepressant
- Uses Venlafaxine hydrochloride is an inhibitor of reuptake of both serotonin (IC50 = 0.21 μM) and norepinephrine (IC50 = 0.64 μM). It is effective in vitro and in vivo and against human as well as rat receptors. As an antidepressant, it is properly placed in the serotonin-norepinephrine reuptake inhibitor class.[Cayman Chemical]
- Uses An inhibitor of ST and SLC6A2.
-
Manufacturing Process
1-[Cyano(-methoxyphenyl)methyl]cyclohexanol
p-Methoxyphenylacetonitrile (50 gm, 0.3 mole) was added to dry tetrahydrofuran (250 ml) and the solution cooled to -70°C under nitrogen. n- Butyl lithium in hexane (210 ml, 0.3 mole) was added dropwise, with stirring. The temperature was maintained below -50°C and a yellow precipitate appeared. After the addition was complete, the reaction mixture was maintained below -50°C for 30 minutes and cyclohexanone (35 ml, 0.3 mole)was added. After a further 45 minutes below -50°C the temperature was allowed to rise to 0°C and a saturated ammonium chloride solution was added. The layers were separated and the aqueous layer extracted with diethyl ether. The combined organic solution was washed with brine, dried over magnesium sulfate and evaporated. The product crystallized (25.2 gm, melting point 125°-127°C). The structure was confirmed by N.M.R. and mass spectral analysis.
1-[2-Amino-1-(p-methoxyphenyl)ethyl]cyclohexanol
1-[Cyano(p-methoxyphenyl)methyl]cyclohexanol (12 g, 0.05 mole) was dissolved on warming in a mixture of ammonia-ethanol (20% v/v, 250 ml) and hydrogenated in a Parr apparatus over 5% rhodium an alumina (2.8 gm). The catalyst was filtered, washed well with ethanol and the combined filtrate evaporated and dried under vacuum yielding an oil (12 gm). Thin layer chromatography: single spot, ninhydrin positive [chloroform-methanol-acetic acid (80:10:10 v/v)].
1-[-2-Dimethyl-amino)-1-(4-methoxyphenyl)-ethyl]cyclohexanol
1-[2-Amino-1-(p-methoxyphenyl)ethyl]cyclohexanol (12 gm; 0.048 mole) was treated with a mixture of formaldehyde (11 ml), formic acid (14.5 ml, 88%) and water (125 ml) and heated at 100°C for five hours. The reaction mixture was cooled and extracted with ethyl acetate. This extract was discarded. The aqueous residue was cooled in ice, rendered basic by the addition of solid potassium hydroxide, saturated with sodium chloride and extracted 3 times with ethyl acetate. The extract was washed with brine, dried over anhydrous potassium carbonate and evaporated to an oily residue (8 gm). This mixture of products was chromatographed on 1 kg of Mallinckrodt Silicar CC7 silica gel and the progress of the chromatography was monitored by thin layer chromatrography using a system comprising ethanol:2 N ammonia:ethyl acetate:cyclohexane 45:8:100:100 (v/v). Fractions containing the desired product were combined and the hydrochloride salt prepared using 4 N HCl in isopropanol. The yield of the free base was 4.6 gm of 1-[(2-dimethylamino)- 1-(4-methoxyphenyl)ethyl]-cyclohexanol. The hydrochloride (venlafaxine): melting point 215°-217°C. The structure was confirmed by mass spectral analysis and N.M.R. analysis. - brand name Effexor (Wyeth).
- Therapeutic Function Antidepressant
- General Description A Certified Snap-N-Spike? Solution suitable for many LC/MS and GC/MS applications from forensic or clinical toxicology analysis to urine drug testing. Also known by the brand name Effexor?, venlafaxine is an SNRI antidepressant approved for the treatment of major depressive and general anxiety disorders.
- Biological Activity Dual serotonin/noradrenalin re-uptake inhibitor that displays ~ 30-fold higher affinity for SERT than NET (K i values are 82 and 2480 nM respectively). Antidepressant; increases swimming and climbing behavior in the forced-swim test in rats.
- Biochem/physiol Actions Venlafaxine is an antidepressant. The mechanism of the antidepresant action of venlafaxine in humans is associated with its potentiation of neurotransmitter activity in the CNS. Venlafaxine is a potent inhibitor of neuronal serotonin and norepinephrine reuptake and weak inhibitor of dopamine reuptake. Venlafaxine has no significant activity for muscarinic, histaminergic, or α-1 adrenergic receptors in vitro. Venlafaxine does not possess MAO inhibitor activity.
- storage Store at RT
Venlafaxine hydrochloride Preparation Products And Raw materials
Raw materials
Preparation Products
Global(667)Suppliers
- Austria 1 Belgium 1 Brazil 3 Canada 6 CHINA 380 Colombia 1 Czech Republic 1 Europe 2 Finland 1 France 2 Germany 8 Greece 1 India 162 Israel 3 Italy 2 Japan 1 Mexico 1 Nepal 1 Nigeria 1 Slovenia 2 South Korea 3 Spain 6 Switzerland 1 United Arab Emirates 1 United Kingdom 14 United States 61 Uruguay 1 Global 667
-
Supplier:
Hebei Chuanghai Biotechnology Co., Ltd
- Tel: +8615531157085
- Email:abby@chuanghaibio.com
- Country:China
- ProdList:8808
- Advantage:58
-
Supplier:
Hebei Yanxi Chemical Co., Ltd.
- Tel: +8617531153977
- Email:allison@yan-xi.com
- Country:China
- ProdList:5854
- Advantage:58
-
Supplier:
Anhui Yiao New Material Technology Co., Ltd
- Tel:+86-18033737140<br/>+86-17354101231
- Email:sales1@hbganmiao.com
- Country:China
- ProdList:227
- Advantage:58
-
Supplier:
Anhui Ruihan Technology Co., Ltd
- Tel: +8617756083858
- Email:daisy@anhuiruihan.com
- Country:China
- ProdList:973
- Advantage:58
-
Supplier:
BEIJING SJAR TECHNOLOGY DEVELOPMENT CO., LTD.
- Tel:+86-18600796368<br/>+86-18600796368
- Email:sales@sjar-tech.com
- Country:China
- ProdList:485
- Advantage:58
-
Supplier:
Nanjing ChemLin Chemical Industry Co., Ltd.
- Tel:025-83697070
- Email:product@chemlin.com.cn
- Country:CHINA
- ProdList:3009
- Advantage:60
-
Supplier:
Nanjing Finetech Chemical Co., Ltd.
- Tel:025-85710122 17714198479
- Email:sales@fine-chemtech.com
- Country:CHINA
- ProdList:885
- Advantage:55
-
Supplier:
Hefei TNJ Chemical Industry Co.,Ltd.
- Tel:+86-0551-65418679<br/>+8618949832763
- Email:info@tnjchem.com
- Country:China
- ProdList:2986
- Advantage:55
-
Supplier:
SHANDONG ZHI SHANG CHEMICAL CO.LTD
- Tel: +86 18953170293
- Email:sales@sdzschem.com
- Country:China
- ProdList:2930
- Advantage:58
-
Supplier:
Hubei Jusheng Technology Co.,Ltd.
- Tel:18871490254
- Email:linda@hubeijusheng.com
- Country:CHINA
- ProdList:28172
- Advantage:58
99300-78-4, Venlafaxine hydrochloride Related Search:
- O-Desmethylvenlafaxine Methoxyammonium chloride Topotecan hydrochloride Ethanol Minocycline 4-(Diethylamino)salicylaldehyde N,N-Dimethylbenzylamine Methylparaben Paraquat dichloride Ethyl acrylate Dimethylamine Methyl acrylate Basic Violet 1 Dimethylamine hydrochloride Acetonitrile Ethyl cellulose Pralmorelin Kresoxim-methyl
- 99300-78-4
- Medicine intermediate
- Active Pharmaceutical Ingredients
- Antidepressant
- Pharmaceuticals
- Intermediates & Fine Chemicals
- Inhibitors
- APIs
- Serotonin receptor
- 化学原料
- 杂质对照品
- 抑制剂
- 配体家族
- 药靶配体
- 临床检测标准物质
- 精细化工原料
- 化工原料
- 化学试剂
- 原料
- 化工中间体
- 化工
- 标准品
- 医药原料药
- 药物杂质及中间体
- 中间体类
- 对照品
- 神经信号
- 小分子抑制剂
- 精神类
- 医药原料
- 化学原药
- 原料药
- 其他化学试剂
- 植物神经系统药物
- 中枢神经系统药物
- 医药与生物化工
- 医药原料和中间体
- C17H27NO2ClH
- C17H27NO2HClC17H28ClNO2
- C17H28ClNO2
- C17H27NO2
- C17H27NO2.HCl
- 9300-78-4
- 00-00-2
- VENLAFAXINE HYDROCHLORIDE 文拉法辛盐酸盐 |CAS 99300-78-4
- 盐酸文拉法辛,10 MM DMSO 溶液
- VENLAFAXINE HYDROCHLORIDE,SEROTONIN-NOREPINEPHRINE REUPTAKE抑制剂
- 乙腈中盐酸文拉法辛
- 盐酸文拉法辛溶液
- 文拉法辛/用于系统适用性试验/A1A
- 文拉法辛(盐酸文拉法辛)检测标准品
- 甲醇中盐酸文拉法辛
- 优选]VENLAFAXINE
- (±)-文拉法辛-[D6]盐酸盐
- 拉法辛盐酸盐
- 甲醇中盐酸文拉法辛溶液,100ΜG/ML
- 盐酸文拉法辛, 一种5-羟色胺-去甲肾上腺素再摄取抑制剂
- 1-[2-(二甲胺)-1-(4-甲氧苯基)乙基]环己醇盐酸盐