Chemical Properties
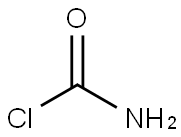
Carbamoyl chloride,
H2N – COCl, is unstable; it may be stabilized by Lewis acids (e.g., aluminum chloride) as adducts
and in this form can be used in the Friedel-Crafts
acylation of arenes. Since many of the
monosubstituted carbamoyl chlorides also deteriorate on storage, the corresponding isocyanates
are used instead. Disubstituted carbamoyl chlorides are stable and can be stored for prolonged
periods.
Physical properties
The simple carbamoyl
chlorides are colorless liquids or solids, usually
with pungent odor.
Uses
Carbamoyl chloride can be used as an intermediate for carbamate pesticides.
Uses
Carbamoyl chlorides are intermediates with many uses. Of primary industrial
importance are the reactions with alcohols, phenols, and oximes to give carbamic acid esters;
with thiols (mercaptans) to give thiocarbamates;
with amines and hydroxylamines to give substituted ureas; and with imidazoles and triazoles to
give carbamoyl azoles.
Definition
ChEBI: Carbamyl chloride is an organonitrogen compound.
Synthesis
Carbamoyl chloride can be synthesized from methylamine and phosgene at 200-240℃.