Description
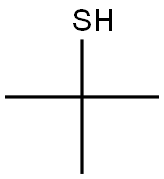
tert-Butylthiol, also known as 2-methyl propane-2-thiol, 2- methyl-2-propane thiol, tert-butyl mercaptan (TBM), and t-BuSH, is an organo sulfur compound with the formula (CH
3)
3CSH. This thiol may have been used as a flavoring agent, as an odorant for natural gas (which is odorless), and also in a wide range of organic reactions.
Chemical Properties
liquid with an exceedingly unpleasant smell
Uses
Tert-Butylthiol is the main ingredient in many gas odorant blends. It is always utilized as a blend of other compounds, typically dimethyl sulfide, methyl ethyl sulfide, tetrahydrothiophene or other mercaptans (isopropyl mercaptan, sec-butyl mercaptan and/or n-butyl mercaptan, due to its rather high melting point of 273 K. These blends are used only with natural gas and not propane, as the boiling points of these blends and propane are quite different. As propane is delivered as a liquid and vaporizes to gas when being delivered to the appliance, the vapor liquid equilibrium would substantially reduce the amount of odorant blend in the vapor.
Tert - Butyl thiol has been listed on the European Food Safety Authority (FL-no: 12.174) as a flavor additive. There is no indication of what flavor or flavors it may have been used in. It has been removed from this list.
Uses
2-Methyl-2-propanethiol was used in reaction of 2-methyl-2-propanethiol on Mo(110) using temperature programmed reaction, high resolution electron enegy loss and X-ray photoelectron spectroscopies. It was used in the synthesis of chain-transfer agents for reversible addition-fragmentation chain-transfer copolymerization of vinylidene chloride and methyl acrylate.
Preparation
tert-Butyl thiol likely does not occur naturally, but at least one publication has listed it as a very minor component of cooked potatoes. The compound was first prepared in 1890 by Leonard Dobbin by the reaction of zinc sulfide and t-butyl chloride.
The compound was later prepared in 1932 by the reaction of the Grignard reagent, t-BuMgCl, with sulfur to give the corresponding thiolate, followed by hydrolysis. This preparation is shown below:
t-BuMgCl + S → t-BuSMgCl
t-BuSMgCl + H
2O → t-BuSH + Mg(OH)Cl
It is currently prepared industrially by the reaction of isobutylene with hydrogen sulfide over a clay (silica alumina) catalyst.
Reactions
tert-Butylthiol can react with metal alkoxides and acyl chlorides to form thiol esters, as shown in the equation :
In the reaction above, thallium (I) ethoxide converts to thallium (I) t-butyl thiolate. In the presence of diethyl ether, thallium (I) tbutylthiolate reacts with acyl chlorides to give the corresponding tertbutyl thioesters. Like other thio esters, it reverts back to tert-butylthiol by hydrolysis.
Lithium 2-methyl propane-2-thiolate can be prepared by treatment of tert-butyl thiol with lithium hydride in an aprotic solvent such as hexa methyl phosphorous triamide (HMPT). The resulting thiolate salt is a useful demethylating reagent. For example, treatment with 7- methyl guanosine gives guanosine. Other N-methylated nucleosides in tRNA are not demethylated by this reagent .
General Description
2-Methyl-2-propanethiol undergoes ring opening nucleophilic reaction with 3-isothiazolones and reaction kinetics studies suggested reaction was second order in thiol and third order overall.
Hazard
Flammable, dangerous fire risk. Very toxic.
Safety Profile
Moderately toxic by
intraperitoneal route. Mildly toxic by
ingestion. An eye irritant. A very dangerous
fire hazard when exposed to heat or flame.
Can react vigorously with oxidizing
materials. To fight fire, use alcohol foam,
dry chemical, mist, fog. When heated to
decomposition or on contact with acid or
acid fumes it emits highly toxic fumes of
SOx.
Safety
Even in well ventilated areas, extreme caution must be made when handling tert-butylthiol as it is a highly odorous chemical with an odor threshold of < 0.33 ppb. Extreme caution is not due to toxicity, but due to the significant odor and concerns that this odor would cause to the many individuals that might be exposed. The PEL for thiols of most types is 500 ppb, primarily due to reaction of nausea at levels of 2–3 ppm. The LC
50 of tert-butylthiol is much, much higher.
Purification Methods
Dry the thiol for several days over CaO, then distil it from CaO. Purify it as for 2-methylpropane-1-thiol above. [Beilstein 1 H 383, 1 II 416, 1 III 1589, 1 IV 1634.]
Metal complexes
The anion derived from tert – butyl thiol forms complexes with various metals. One example is tetra kis (tert-butyl thiolato ) molybdenum (IV), Mo(t-BuS)
4. This complex was prepared by treating MoCl
4 with t-BuSLi :
Mo Cl
4 + 4t-Bu S Li → Mo (t-BuS)
4 + 4LiCl
Mo(t-BuS)
4 is a dark red diamagnetic complex that is sensitive to air and moisture. The molybdenum center has a distorted tetra hedral coordination to four sulfur atoms, with overall D2 symmetry.