Chemical Properties
yellow to green-yellow powder
Uses
Pyranine is used as a fluorescent pH indicator with emission change in the physiological pH range. It acts as a dye in optical sensor applications. It finds application as a coloring agent and biological stain. It is also used in the measurement of pH of intracellular fluid in the cells.
Preparation
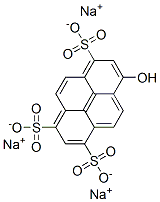
commonly known as Pyranine. Pyrene-1,3,6,8-tetrasulfonic acid?and 15 ~ 20% sodium hydroxide reflux, also can use ammonia water on hydrolysis reaction, this condition the product contains APTS(8-Aminopyrene-1,3,6-trisulfonic acid?).
Definition
ChEBI: Pyranine is an organic sodium salt. It has a role as a fluorochrome. It contains a pyranine(3-). It derives from a hydride of a pyrene.
Flammability and Explosibility
Not classified
Biological Activity
Pyranine (HPTS; Solvent Green 7) is a pH-sensitive fluorescent indicator. Pyranine is a class of fluorescent chemosensors for Cu+ ions (λex=450 nm, λem=510 nm).
in vitro
Pyranine is capable of discriminating ranges of cations from the Cu + ion, even in competing environment. Pyranine displays a rapid fluorescence response (t 1/2 =1.66 min) towards the Cu + ion, and the micromolar detection limit enables the detection of the ion in environmental samples. The observed stoichiometry of complexation between Pyranine and Cu + is 2:1.The pH-sensitive fluorescent indicator Pyranine is studied to determine its usefulness as probes for the living cornea and anterior chamber. Adequate concentrations are reached in the cornea and anterior chamber after topical administration; measurements can be made by fluorophotometry for many hours. The pH is calculated by measuring the ratio of fluorescent intensity at two excitation wavelengths, I463/I404, a measurement which is dependent on pH but independent of the concentration of the fluorophore and other variables which can alter the intensity. In the rabbit eye, Pyranine in the cornea and anterior chamber is observed to undergo easily measurable changes in fluorescent ratios associated with lid closure and contact lens wear, indicating its sensitivity to mild changes in pH.
Properties and Applications
yellow green. Slightly soluble in glacial acetic acid, 30% hydrochloric acid, insoluble in acetic acid and 10% hydrochloric acid. Mainly used in medicine and cosmetics coloring.
|
Standard
|
Light Fastness
|
Heat-resistant(℃)
|
water
|
Sodium Carbonate(5%)
|
Hydrochloric acid(5%)
|
|
Melting point
|
Stable
|
|
ISO
|
|
|
|
Dissole
|
|
|
Purification Methods
Purify the salt by chromatography with an alumina column, and elute with n-propanol/water (3:1, v/v). Recrystallise it from aqueous acetone (5:95, v/v) using decolorising charcoal. [Beilstein 1 III 565.] IRRITANT.