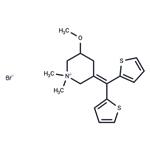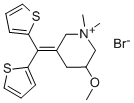
Timepidium bromide
- Product NameTimepidium bromide
- CAS35035-05-3
- CBNumberCB9308839
- MFC17H22BrNOS2
- MW400.4
- MDL NumberMFCD00865193
- MOL File35035-05-3.mol
Chemical Properties
| Melting point | 198-200° |
| storage temp. | Store at -20°C |
| solubility | Soluble in DMSO |
| FDA UNII | 8R9E4766V4 |
| ATC code | A03AB19 |
Timepidium bromide Price
| Product number | Packaging | Price | Product description | Buy |
|---|---|---|---|---|
| American Custom Chemicals Corporation CHM0304658 | 5MG | $497.67 | 1,1-DIMETHYL-5-METHOXY-3-(DITHIEN-2-YLMETHYLENE)PIPERIDINIUM BROMIDE 95.00% |
Buy |
Timepidium bromide Chemical Properties,Usage,Production
Originator
Sesden,TANABE SEIYAKU,Japan,1976Definition
ChEBI: Timepidium bromide is an organic molecular entity.Manufacturing Process
120 g of 5-hydroxynicotinic acid are dissolved in 1 liter of methanol. After saturating with dry-hydrogen chloride gas at 0°C, the solution is refluxed for 2 hours. Then, the solution is concentrated to dryness. The residue thus obtained is dissolved in water. The solution is neutralized with sodium bicarbonate. The precipitated crystals are collected by filtration, washed with water and then dried. 126 g of methyl 5-hydroxynicotinate are obtained. Yield: 93%. Melting point 184°C to 186°C.460 g of methyl 5-hydroxynicotinate and 621 g of potassium carbonate are suspended in 200 ml of tetrahydrofuran-methanol (4:1). 1,134 g of dimethyl sulfate are added dropwise to the suspension in nitrogen atmosphere at room temperature. The mixture is stirred overnight at the same temperature and then filtered. The filtrate is concentrated to dryness. The residue thus obtained is mixed with 1.6 liters of methanol and 280 ml of Raney-nickel, and hydrogenated overnight in an autoclave at room temperature and at a pressure of 85 atmospheres. 200 g of Raney-nickel are added to the reaction mixture. The mixture is adjusted to pH 9.5 with triethylamine, and is further subjected to hydrogenation for 20 hours in an autoclave at 70°C and at a pressure of 100 atmospheres. Potassium carbonate and a small amount of ice are added to the reaction mixture to bring the pH to 11. The mixture is extracted with ether. After drying, the ether layer is filtered. The filtrate is evaporated to remove ether. The residue thus obtained is distilled under reduced pressure. 450 g of methyl N-methyl-5-methoxynipecotinate are obtained. Yield: 80%. Boiling point 80°C to 81°C/0.5 mm Hg. A solution of 18 g of 2-thienyl bromide in 30 ml of tetrahydrofuran is gradually added to a mixture of 2.6 g of magnesium and 80 ml of tetrahydrofuran under stirring at 50°C. The mixture is stirred for 5 hours at room temperature until the magnesium is entirely dissolved in the solution. 6.2 g of methyl N-methyl-5-methoxy-nipecotinate are added to the mixture. Then, the mixture is refluxed for 4 hours. After the reaction is completed, tetrahydrofuran is distilled off under reduced pressure. An aqueous ammonium chloride solution is added to the residue, and the solution is extracted with chloroform. The extract is dried and then evaporated to remove chloroform. The viscous oil thus obtained is recrystallized from a mixture of benzene and ether. 7 g of di-(2-thienyl)-(N-methyl-5-methoxy-3-piperidyl)- carbinol are obtained as crystals. Melting point 142°C to 146°C. 7 g of the product are dissolved in 150 ml of 10% hydrochloric acid, and the solution is heated at 80°C for 30 minutes. After the reaction is completed, the solution is basified with sodium hydroxide and then extracted with ether. The extract is washed with water, dried and evaporated to remove ether. 5 g of di- (2-thienyl)-(N-methyl-5-methoxy-3-piperidylidene)-methane are obtained as pale yellow oil.
365 mg of di-(2-thienyl)-(N-methyl-5-methoxy-3-piperidylidene)-methane are dissolved in 15 ml of ether. 1 ml of methyl bromide is added to the solution. Then, the solution is stirred overnight. The precipitated crystals are collected by filtration and recrystallized from a mixture of acetone and ether. 390 mg of di-(2-thienyl)-(N-methyl-5-methoxy-3-piperidylidene)-methane methyl bromide are obtained as colorless crystals. Melting point 198°C to 200°C.
Therapeutic Function
Anticholinergicin vivo
Effects of Timepidium bromide (TB), acetylcholine (ACh) and neostigmine (Neost) on gastric and duodenal blood flow distribution are studied by the use of 131 I-labeled macroaggregated human serum albumin (MAA) in rabbits. In normal rabbits, gastric blood flow is found to be uneven in various regions of the stomach: anterior corpus (50% of total gastric blood flow) greater than posterior corpus (40%) greater than pyloric antrum (7%). Intravenous administration of Timepidium bromide (200 μg/kg) to normal rabbits produces a slight increase in total gastric blood flow, but the increase in the mucosal layer of the pyloric antrum is considerable.Preparation Products And Raw materials
Timepidium bromide Suppliers
Global(28)Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| +8618523575427 | sales@conier.com | China | 49732 | 58 | |
| +1-781-999-5354 +1-00000000000 |
marketing@targetmol.com | United States | 32159 | 58 | |
| support@targetmol.com | United States | 38630 | 58 | ||
| +8613564774135 | zijue.cai@tsbiochem.com | United States | 19881 | 58 | |
| 021-58955995 | sales@medchemexpress.cn | United States | 4861 | 58 | |
| 15911056312 | liming@bio-fount.com | China | 9729 | 58 | |
| 0531-69900633 18340090523 |
changyuan20@163.com | China | 2717 | 58 | |
| 13348960310 13348960310 |
3003867561@qq.com | China | 10524 | 58 | |
| 010-60605840 15801484223; |
psaitong@jm-bio.com | China | 29774 | 58 | |
| 4008200310 | marketing@tsbiochem.com | China | 24641 | 58 |
35035-05-3, Timepidium bromideRelated Search
1of4
PROMPT×
PROMPT
The What'sApp is temporarily not supported in mainland China
The What'sApp is temporarily not supported in mainland China
Cancel
Determine