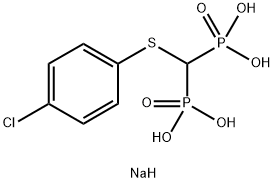
Description
Tiludronate disodium, the first of the third generation of bisphosphonates, was
introduced to the market in Switzerland for the treatment of Paget‘s disease.
Bisphosphonates have high affinity for bone. When given orally, they are not
metabolized but are absorbed, stored, preferentially localized to the skeleton where
they inhibit bone resorption and therefore are useful in the treatment of diseases of high
bone turnover such as Paget’s disease and hypercalcemia of malignancy.
Mechanistically, tiludronate disodium is suggested to be a specific inhibitor of
functioning osteoclasts through selective incorporation into the polarized osteoclastlike
multinucleated cells and direct interference with the maintenance of the
cytoskeletal structure. Tiludronate disodium is reportedly in clinical trials for
osteoporosis in post-menopausal women.
Chemical Properties
Fine White to Off-White Crystalline Powder
Originator
Sanofi (France)
Uses
A biphosphonate bone resorption inhibitor.
Manufacturing Process
The 50% strength suspension of sodium hydride in oil is added, a little at a
time to a solution of tetraisopropyl methylene-diphosphonate in
dimethylformamide. After all has been added, the mixture is stirred, the 1-(4-
chlorphenyl) disulfide is then added and the whole is heated at 25°C for 6 h.
The mixture is evaporated to dryness in vacuo and residue is taken up in
hexane. The solution is washed with water and dried. The solvent is
evaporated to dryness and the residue is chromatographed on silica column,
elution being carried out with a 98:2 (v/v) mixture of methylene
chloride/methanol. The obtained 1-(4-chlorphenylthio)tetraisopropyl
methylene-diphosphonate is then hydrolyzed with 12 N HCl for 18 h to give
the 1-(4-chlorphenylthio)methylene-diphosphonic acid.
The 1-(4-chlorphenylthio)methylene-diphosphonic acid are dissolved in water,
containing sodium hydroxide. The solution is filtered, the methanol are then
added and the mixture is left to crystallize. The precipitate is filtered off and
washed with methanol and dried at 80°C in vacuo and the disodium salt of 1-
(4-chlorphenylthio)methylene-diphosphonic acid is thus obtained.
Therapeutic Function
Bone calcium regulator
Biological Activity
Tiludronate (Tiludronic Acid) disodium is an orally active bisphosphonate that acts as a bone modulating agent. Tiludronate disodium for the study of metabolic bone diseases. Tiludronate disodium is a potent inhibitor of osteoclast vacuolar H(+)-ATPase.
in vitro
The ability of Tiludronate to inhibit proton transport is 5-fold higher in kidney-derived vesicles (IC 50 =1.1 mM) and 10,000-fold higher in vesicles derived from osteoclasts (IC 50 =466 nM). Tiludronate also potently inhibits proton transport in yeast microsomal preparations (IC 50 =3.5 microM) and inhibits the activity of purified yeast V-ATPase. The inhibition of the osteoclast V-ATPase-mediated proton transport by Tiludronate is rapid, pH-dependent, and reversible.
in vivo
Tiludronate exerts a dose-dependent inhibitory activity on bone resorption. Tiludronate could act on mature osteoclasts by reducing their capacity to secrete proton into the resorption space and also by favoring their detachment from the bone matrix. Tiludronate is also tested in other models of osteoporosis. In the castrated male rat model, Tiludronate (5-200 mg/kg; p.o.) prevents the decrease in the skeletal mass, assessed physically by measuring the bone weight and density or chemically by determining the calcium and phosphate content.