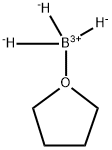
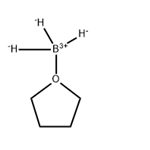
Description
Borane tetrahydrofuran complex (BH3-THF) is widely used as a reducing agent in organic synthesis. It is also used as a reagent in hydroboration reactions.It is a charge-transfer complex that is a useful surrogate for diborane1 in organic synthesis. It can be used to reduce carboxylic acids to alcohols or nitriles to primary amines. It reacts with olefins to add the BH2 functional group. Alkyl- or arylboranes formed in this way can further react with unsaturated compounds such as olefins, imines, ketones, and alkynes (the hydroboration reaction) to make useful boron-containing intermediates.
Chemical Properties
colourless liquid
Uses
Borane-tetrahydrofuran complex is used to reduce Nylon surface amide groups to secondary amines.It is an important reagent used in the reduction of certain functional groups viz. aldehyde, ketone, carboxylic acid, amide, oxime, imine and nitrile. It is also used as hydro borating agent. It acts as a borane source for oxazaborolidine catalyzed asymmetric reductions.
Uses
Borane-tetrahydrofuran complex is an important reagent used in the reduction of certain functional groups viz. aldehyde, ketone, carboxylic acid, amide, oxime, imine and nitrile. It is also used as hydro borating agent. It acts as a borane source for oxazaborolidine catalyzed asymmetric reductions. It is utilized to reduce nylon surface amide groups to secondary amines.
Application
New, Safer, NIMBA-Stabilized BH3 THF Solutions
BH3-THF can be used as a reducing agent for the reduction of various functional groups such as carboxylic acids, aldehydes, ketones, esters, acid chlorides, nitriles, epoxides, amides, lactones, oximes, and imines into corresponding alcohols and amines. Grignard reagents, arylmercury, arylthalium, and allyl and propargyllithium compounds react with BH3?THF to give organoboranes, which can be oxidized to the corresponding alcohols, phenols, and 1,3-diols.
It can also be used:
To synthesize the chiral borane catalyst, which is used in the enantioselective halo-aldol reaction.
To prepare 9-unsubstituted acridines by reduction of corresponding acridones.
To reduce nylon surface amide groups to secondary amines.
Reactions
Borane-tetrahydrofuran complex (BTHF) is a valuable reagent for the reduction of functional groups and for hydroboration reactions with carbon-carbon double and triple bonds. Functional groups that are readily reduced by BTHF include aldehyde, ketone, carboxylic acid, amide, oxime, imine, and nitrile. The carboxylic acid group is reduced at a faster rate than most groups including non-conjugated alkene. Conjugated α,β-unsaturated carboxylic acids give saturated alcohols as the major products.
Ketones and the carbonyl of enones are effectively reduced with borane-tetrahydrofuran. The addition of borohydride to the reaction solution is advantageous for accelerated reduction as well as higher selectivity towards carbonyl reduction in conjugated and non-conjugated enones.
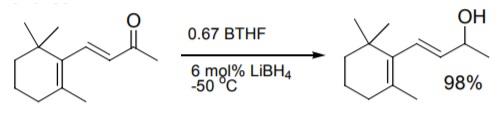
Asymmetric ketone reduction using chiral oxazaborolidine catalysts was recently reviewed. Work at Callery with BTHF improved on reaction conditions to provide consistent results in the reduction.
General Description
Borane tetrahydrofuran complex (BH
3-THF) is widely used as a reducing agent in organic synthesis. It is also used as a reagent in hydroboration reactions.
reaction suitability
reagent type: reductant
Precautions
Air and moisture sensitive. Forms explosive peroxides in contact with air. Incompatible with acids, acid chlorides, acid anhydrides, oxidizing agents and alcohols. On hydrolysis, it forms hydrogen and boric acid.