Description
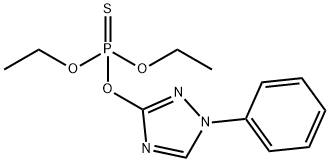
Triazophos is a Yellowish-brown-oil chemical pesticide used as an insecticide, nematicide and acaricide. The chemical is an organophosphorus compound and ingestion and other exposures to the chemical can cause various symptoms. The type and severity of symptoms varies depending on the amount of chemical involved and the nature of the exposure. The chemical may be absorbed through the skin. More detailed information about the symptoms, causes, and treatments of Chemical poisoning--Triazophos is available below. It is stable to sunlight and its molecular weight313.312 g/mol. For solublity, It solubles in organic solvents and in acetone, dichloromethane, methanol, isopropanol, ethyl acetate and plyethyleneglycols >500; n-hexane 11.1 (all in g/L at 20 degree).
Methods of Manufacturing
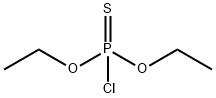
Triazophos is produced by reaction of 1-phenyl-3-hydroxy-1H-1,2,4-triazole, suspended in acetone, with diethoxythiophosphoryl chloride in the presence of triethylamine.
Physical and chemical properties
The pure product is a pale yellow-brown oily liquid; melting point: 0-5 °C; vapor pressure: 0.39 Mpa; relative density: 1.24; it decomposes at 185 °C before reaching the boiling point and can be degraded by acids and alkalis.
Uses
Triazophos is a broad-spectrum insecticide and miticide and has a certain degree of nematicidal effects. It has excellent preventive effects on many pests of the major crops including grain, cotton and vegetables, such as stem borers, rice planthoppers, spider mites, cotton bollworms, nematodes, and it is especially effective on the lepidoptera.
Storage and transportation
It should be protected from the sun, rain and heat sources and kept away from food, seeds and feeds.
References
1.http://www.sld.chemchina.com/slden/cpyfw/ppysb/jscg/webinfo/2012/06/1342611935442261.htm
2.https://pubchem.ncbi.nlm.nih.gov/compound/Triazophos
3.https://fr.wikipedia.org/wiki/Triazophos
Uses
An organophosphorus insecticide.
Uses
Triazophos is used to control chewing and sucking insects and
mites in a wide range of crops. It is also used to control some nematodes
in ornamentals and strawberries.
Definition
ChEBI: Triazophos is an organic thiophosphate and an organothiophosphate insecticide. It has a role as an EC 3.1.1.7 (acetylcholinesterase) inhibitor, an acaricide, an agrochemical and a nematicide. It is functionally related to a 1-phenyl-1H-1,2,4-triazol-3-ol.
General Description
Yellowish oil. Used to control insects, mites, and nematodes. Not registered as a pesticide in the U.S.
Reactivity Profile
Organophosphates, such as Triazophos, are susceptible to formation of highly toxic and flammable phosphine gas in the presence of strong reducing agents such as hydrides. Partial oxidation by oxidizing agents may result in the release of toxic phosphorus oxides.
Health Hazard
This is an organophosphate pesticide. Triazophos is a cholinesterase inhibitor and acts on the central nervous system. Organic phosphorus insecticides are absorbed by the skin as well as by the respiratory and gastrointestinal tracts.
Fire Hazard
This is an organophosphate pesticide. Some of these materials may burn but none of them ignite readily. Container may explode in heat of fire. Fire may produce irritating or poisonous gases. Degraded by acids and alkalis.
Agricultural Uses
Insecticide: Triazophos is a broad-spectrum insecticide and acaricide used to control sucking and chewing pests on a variety of crops, including cotton, rice, corn, beets and fruit
trees. Not currently registered for use in the U.S. Not approved for use in EU countries
. There are more than 30
global suppliers
.
Trade name
HILAZPOPHOS®; HOE 2960 OJ®; HOE
002960®; HOSTATHION®; SUTATHION®
Metabolic pathway
The main route of biotransformation of triazophos is degradation to 1-
pheny1-3-hydroxy-1,2,4-triazole, probably either via hydrolysis of triazophos
oxon or through oxidative cleavage of triazophos itself. The triazole
ring of this metabolite is opened to give 1-phenylsemicarbazide and semicarbazide,
both of which are conjugated in the rat and also degraded to
urea as the main urinary metabolite.
Degradation
Triazophos is stable to light but is hydrolysed by acids and alkalis (PM).