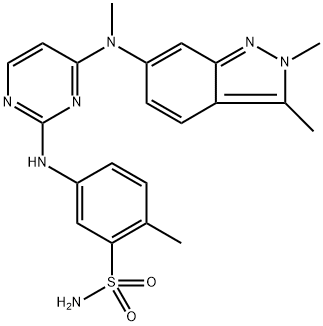
product description
Pazopanib(444731-52-6) is researched and developed by a GlaxoSmithKline ,it is a novel oral vascular generation inhibitor which may interfere stubborn tumor survival and new blood vessels generation growth needed .Targeting vascular endothelial growth factor receptor (VEGFR),it works by inhibiting angiogenesis of tumor blood supply.it is suitable for advanced renal cell carcinoma ( a discovery of cancer cells in the renal tubules), soft tissue sarcoma (STS), epithelial ovarian cancer and non-small cell lung cancer (NSCLC) treatment.
Minnesota Rochester, Mayo Clinical Research Clinical Research Center researches show that pazopanib (trade name Votrient) can effectively cure thyroid cancer,and can make half of the patients tumor volume reduced, compared to the commonly used drugs ,it is more prolonged stable ,and can be maintained for a considerable period of treatment, the drug has the highest response rate in treatment of thyroid cancer so far.
The US Food and Drug Administration (FDA) approved on October 19, 2009 that pazopanib (trade name Votrient) is listed ,the sixth approved drug treatment of kidney cancer since 2005. In 2009, approximately 49,000 patients were diagnosed with renal cell carcinoma, 11,000 patients died of the disease.
June 15, 2010, pazopanib, EU conditionally approved it for advanced renal cell cancer and first-line treatment in patients with previously received cytokine therapy. Once the European Commits to give final marketing authorization for this drug, GlaxoSmithKline will have exclusive marketing rights pazopanib for ten years.
The efficacy of the treatment of advanced renal cell carcinoma
In order to investigate the efficacy of molecule targeting drug tyrosine kinase inhibitor pazopanib which inhibist the angiogenesis in patients with advanced renal cell carcinoma, People's Liberation Army General Hospital, in June 2006 to May 2007, the participation of pazopanib in patients with advanced renal cell carcinoma randomized clinical drug trials of outpatient 14 cases, were divided into pazopanib group (n = 10) and placebo group ( 4 cases), were treated with pazopanib 800mg/d and placebo after 12 weeks of treatment duration, according to the results of CT scan before and after medication , efficacy was determined .
The results showed that the reduced average ratios of measurable disease focus of pazopanib and placebo patients were 27.6%,-2.8% (P <O.05); the disease control rate of pazopanib and placebo groups were 100% and 25%, respectively. Conclusions confirmed that molecule targeting drug tyrosine kinase inhibitor pazopanib which inhibist the angiogenesis can have a significant therapeutic effect and high security on metastatic renal cell carcinoma in a short period of time.
A clinical trail study phase I showed that compared with placebo, pazopanib can reduce the risk of death and tumor progression by 54%. Overall,in the product group of patients ,disease progression-free survival (PFS) mean survival time was 9.2 months ,in the placebo group was 4.2 months. In previously treated patients who had not received in this product group average survival time was 11.1 months in the placebo group was 2.8 months; previously received cytokine therapy patients, pazopanib group survived an average of 7.4 months, the placebo group was 4.2 months.
The above information is edited by the chemicalbook of Tian Ye.
Adverse reactions
Diarrhea, hypertension, hair color change, nausea, loss of appetite, vomiting, fatigue, weakness, headache, abdominal pain and so on.
Cause serious liver problems, so health care workers should take blood tests to monitor liver function before using the drug in patients and during treatment of patients.
Hazardous to the fetus,people during pregnancy are forbid using this drug.
Pazopanib can also lead to arrhythmias, during treatment patients should have regular electrocardiogram measuring heart rate, and regular blood tests to monitor electrolytes since an electrolyte imbalance will also play a role in arrhythmia.
Description
Pazopanib is a multi-kinase inhibitor that inhibits the VEGF receptors VEGFR1, VEGFR2, and VEGFR3 (IC
50s = 10, 30, and 47 nM, respectively, in a cell-free enzyme assay). It also inhibits PDGFRα, PDGFRβ, and c-Kit (IC
50s = 71, 84, and 74 nM, respectively, in a cell-free enzyme assay) as well as additional receptor tyrosine kinases. Pazopanib inhibits upregulation of the surface adhesion proteins ICAM-1 and VCAM-1 induced by VEGF in multiple myeloma cells cocultured with human umbilical vein endothelial cells (HUVECs) and decreases multiple myeloma cell adhesion to HUVECs. It also inhibits proliferation of multiple myeloma cells cocultured with HUVECs. Pazopanib (30 and 100 mg/kg) reduces tumor growth, induces apoptosis, decreases angiogenesis, and increases survival in a multiple myeloma mouse xenograft model. Formulations containing pazopanib have been used in the treatment of cancer.
Chemical Properties
Off-White Solid
Characteristics
Class: receptor tyrosine kinase
Treatment: RCC, STS
Oral bioavailability = 14–39%
Elimination half-life = 31 h
Protein binding > 99.9%
Uses
Pazopanib Hydrochloride (GW786034, Votrient, Armala) is a novel multi-target inhibitor of VEGFR1, VEGFR2, VEGFR3, PDGFR, FGFR, c-Kit and c-Fms with IC50 of 10 nM, 30 nM, 47 nM, 84 nM, 74 nM, 140 nM and 146 nM, respectively.
Uses
An oral angiogenesis inhibitor targeting VEGFR and PDGFR.
Uses
Pazopanib is a novel multi-target inhibitor of VEGFR1, VEGFR2, VEGFR3, PDGFR, FGFR, c-Kit and c-Fms with IC50 of 10 nM, 30 nM, 47 nM, 84 nM, 74 nM, 140 nM and 146 nM, respectively.
Uses
Pazopanib is a potent and selective multi-targeted receptor tyrosine kinase inhibitor of VEGFR1, VEGFR2, VEGFR3, PDGFR-α/β, and c-Kit.
Definition
ChEBI: A pyrimidine that is 5-(pyrimidin-2-yl}amino-2-methylbenzenesulfonamide substituted at position 4 by a (2,3-dimethylindazol-6-yl)(methyl)amino group. Used as its hydrochloride salt for treatment of kidney cancer.
Clinical Use
Tyrosine kinase inhibitor:
Treatment of metastatic renal cell carcinoma and soft
tissue sarcoma
Drug interactions
Potentially hazardous interactions with other drugs
Antibacterials: avoid with clarithromycin, rifampicin
and telithromycin.
Antifungals: avoid with itraconazole, ketoconazole
and voriconazole.
Antipsychotics: avoid with clozapine (increased risk
of agranulocytosis).
Antivirals: avoid with atazanavir, boceprevir,
indinavir, ritonavir and saquinavir.
Grapefruit juice: avoid concomitant administration.
Avoid concomitant use with other inhibitors or
inducers of CYP3A4. Dose alterations may be
required.
Metabolism
Metabolism primarily by CYP3A4, with minor
contributions from CYP1A2 and CYP2C8. The four
principle pazopanib metabolites account for only 6% of
the exposure in plasma. One of these metabolites inhibits
the proliferation of VEGF-stimulated human umbilical
vein endothelial cells with a similar potency to that
of pazopanib, the others are 10- to 20-fold less active.
Therefore, activity of pazopanib is mainly dependent on
parent pazopanib exposure.
Elimination is mostly via the faeces.