Chemical Properties
GREEN TO DARK GREEN CRYSTALS OR CRYST. POWDER
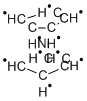
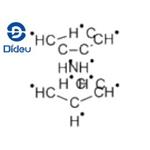
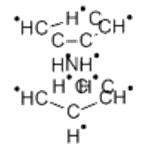
Chemical Properties
Nickelocene is a solid crystalline substance of dark green
color, insoluble in water or carbon tetrachloride, slightly
soluble in alcohol and liquid ammonia, and readily soluble in
nonpolar organic solvents.
Uses
Nickelocene is obtained through reacting nickel halides with
sodium cyclopentadienide. It is used as a catalyst or as a
complexing agent.
Uses
Catalyst, complexing agent.
Uses
This metal alkyl may be used to synthesize hybrid organic-inorganic polymeric molecular layer deposition (MLD). Bis(cyclopentadienyl) nickel (Cp 2 Ni) may be used as a Ni precursor for the atomic layer deposition (ALD) of NiO/carbon nanotubes hybrids.
General Description
Dark liquid in an 8-10% solution in toluene. Insoluble in water.
Air & Water Reactions
Highly flammable. The neat material is sensitive to air. . Insoluble in water.
Reactivity Profile
Organometallics, such as NICKELOCENE, are reactive with many other groups. Incompatible with acids and bases. Organometallics are good reducing agents and therefore incompatible with oxidizing agents. Often reactive with water to generate toxic or flammable gases.
Hazard
Toxic by inhalation and skin contact, a carcinogen (OSHA).
Health Hazard
ACUTE/CHRONIC HAZARDS: NICKELOCENE can be absorbed through the skin.
Fire Hazard
NICKELOCENE is flammable.
reaction suitability
core: nickel
reagent type: catalyst
Safety Profile
Confirmed carcinogen
with experimental carcinogenic,
neoplastigenic, and tumorigenic data. Poison
by intraperitoneal and intramuscular routes.
Moderately toxic by ingestion. When heated
to decomposition it emits acrid smoke and
irritating fumes. See also NICKEL
COMPOUNDS.
Purification Methods
Dissolve the complex in Et2O, filter and evaporate in a vacuum. Purify it rapidly by recrystallisation from pet ether using a solid CO2/Me2CO bath, m 171-173o(in an evacuated tube). Also purify it by vacuum sublimation. [Wilkinson et al. J Am Chem Soc 76 1970 1954, Beilstein 16 IV 1831.]