Nickelocene: Chemical Properties and Uses
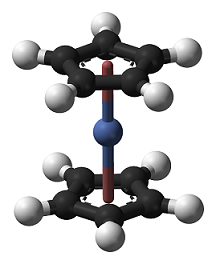
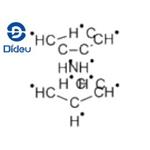
Nickelocene, also known as dicyclopentadienyl nickel, is a dark green crystal. Due to the filling electrons in the anti-bond orbital of the molecular orbital, the structure is unstable, and it slowly decomposes in the presence of air and light, and turns orange C10H10Ni+ after being oxidized. Melting point: 171 ~ 173 ° C; insoluble in water, soluble in most organic solvents such as ether, benzene and tetrahydrofuran; diamagnetic. Like the ferrocene, it has a sandwich structure in which the oxidation number of nickel is +2. Nickelocene, which is very active, decomposes rapidly in the air, and also decomposes in acetone, ether and ethanol.
Nickelocene can be prepared by reacting nickel bromide with sodium cyclopentadienide NaC5H5. It can be used as an anti-vibration agent and catalyst for fuel; nickel monocyclopentadiene and nickel biscyclopentadienyl, which can be used as hydrocarbon cyanide.
The basic raw materials of derivatives, hydrocarbon refining catalysts, hydrogenation catalysts, radical polymerization reagents, vulcanization accelerators and fuel anti-vibration agents. It is also used for nickel plating, preparation of high-purity nickel, and catalysts for polymerization of alkyne etc.. This product has toxicity, inhalation or skin contact is harmful.
Nickelocene is a widely studied organotransition metal complex due to its interesting chemical properties. Nickelocene is an moderately air sensitive organometallic complex that has 20 valence electrons and most of its reactions involve a reduction of its electron count to 18 valence electrons. Nickelocene is also paramagnetic. Nickelocene is usually prepared by reaction an alkali metal cyclopentadienide, prepared from an alkali metal and cyclopentadiene with an anhydrous nickel(II) salt, figure 1. Cyclopentadiene is unstable at room temperature, rapidly undergoing a Diels-Alder reaction with its self to produce the dimer, dicyclopentadiene. Dicyclopentadiene is used as a source of cyclopentadiene by heating it in a distillation apparatus, which causes it to undergo a retro Diels-Alder reaction to form cyclopentadiene. Cyclopentadiene can also be deprotonated by potassium hydroxide in dimethylsulfoxide. The nickel salt still has to be dissolved in an ethereal solvent, 1,2-dimethoxyethane being the best. Diethyl ether also works, but the yields suffer, this would be the most OTC procedure since the only reagent that is hard to find is dicyclopentadiene. The synthesis described involves the use of sodium to form the cyclopentadienide ion and tetrahydrofuran as a solvent.
Ni(O) is a tetracoordinate tetrahedral complex, and the most important is tetracarbonyl nickel [Ni(CO)4] which is the earliest found metal carbonyl complex and used in the manufacture of high purity nickel. Nickel carbonyl is toxic and inhalation should be avoided. When [Ni(CO)4] reacts with nickel (Ni(C5H5)2) at 80 °C, purple-red [Ni(CO)2(C5H5)2] can be obtained. If the reaction happens at high temperature, then the greenish brown [Ni3(CO)2(C5H5)3] can be produced. These are all complex compounds containing intermetallic bonds and carbonyl (CO) bridges and are used as raw materials in the manufacture of various organic nickel compounds.
References
Akhtar, M.H. (1985) Progress in Pesticide Biochemistry and Toxicology, Vol. 4 (Eds.
Hutson, D.H. and Roberts, T.R.), Wiley, Chichester, UK, pp. 238-242.
Arunachalam, K.D. and Lakshamanan, M. (1990) Soil Biol. Biochem., 22,407-412.
Ashworth, R.J. and Sheets, T.J. (1972) J. Agric. Food Chem., 20,407-412.
Chio, H. and Metcalf, R.L. (1979) J. Econ. Entomol., 72,732-738.
Dorough, H.W. (1968) J. Agric. Food Chem., 16,319-325.
Fuhremann, T.W. and Lichtenstein, E.P. (1980) J. Agric. Food Chem., 28,446-452.
Head, I.M., Cain, R.B., Suett, D.L. and Walker, A. (1988) Proc. Brit. Crop Prot.
Con$ - Pests and Diseases, 2,699-704.
See also
Lastest Price from NICKELOCENE manufacturers

US $120.00/kg2025-04-15
- CAS:
- 1271-28-9
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20ton
US $0.00/kg2025-03-03
- CAS:
- 1271-28-9
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10000KGS