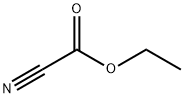
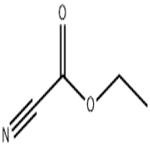
Chemical Properties
Clear Colourless Liquid
Uses
Ethyl cyanoformate can be used as a cyanide source for the cyanation of:
- Activated olefins in the presence of a titanium catalyst.
- Carbonyl compounds catalyzed by 4-dimethylaminopyridine (DMAP) to yield corresponding cyanohydrin carbonates under metal-free and solvent-free conditions.
- Aldehydes and 2,2,2-trifluoroacetophenone catalyzed by N-heterocyclic carbene (NHC) to yield corresponding cyanohydrins ethyl carbonates.
Uses
Ethyl Cyanoformate (cas# 623-49-4) is a compound useful in organic synthesis.
Uses
Ethyl cyanoformate is used as reagent in the preparation of N-substituted amindinoformic acid and ethyl-4-quinazoline -2-carboxylate.
Application
Ethyl cyanoformate can be used as a cyanide source for the cyanation of:
Activated olefins in the presence of a titanium catalyst.
Carbonyl compounds catalyzed by 4-dimethylaminopyridine (DMAP) to yield corresponding cyanohydrin carbonates under metal-free and solvent-free conditions.
Aldehydes and 2,2,2-trifluoroacetophenone catalyzed by N-heterocyclic carbene (NHC) to yield corresponding cyanohydrins ethyl carbonates.
Ethyl cyanoformate is a general cyanating agent. It can be used in the nucleophilic addition of cyanide to carbonyl compounds, oxidative cyanation of tertiary amines and in the synthesis of β-cyano-substituted acrylates from alkynes.
Purification Methods
Dissolve the cyanoformate in Et2O, dry it over Na2SO4, filter, evaporate and distil it [Malachowsky et al. Chem Ber 70 1016 1937, Adickes et al. J Prakt Chem [2] 133 313 1932, Grundmann et al. Justus Liebigs Ann Chem 577 77 1952]. [Beilstein 2 IV 1862.]
Precautions
Moisture sensitive. Keep the container tightly closed in a dry and well-ventilated place. Incompatible with strong acids, strong bases, strong oxidizing agents and strong reducing agents.