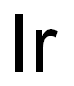Description
Iridium metal was detected in the black residue of aqua regia extract of platinum and identified as an element by British chemist Smithson Tennant in 1803. Around the same time, existence of this new metal was proposed by Vauquelin and deFourcroy in France in the course of their extraction of platinum by aqua regia. Tennant named this element Iridium after the Greek word, Iris, meaning rainbow.
Iridium occurs in small amounts in native platinum or platinum metal alloys. Iridium and osmium together constitute “osmiridium,” which is resistant to chemical attack and is a byproduct of platinum extraction.
Chemical Properties
Iridium (CASRN 7439-88-5) is a hard, brittle, yellowish- white metal that can withstand high temperatures and has a BP of 4428 °C (Lide, 2006). It is the most corrosion resistant metal known (Lide, 2006). Its resistance to high temperatures makes it attractive for making crucibles and other apparatuses that must withstand extreme temperatures. Iridium may be the densest element discovered (Lide, 2006).

Uses
The most important use of iridium is as an alloying metal for platinum and palladium. Such alloys are used for jewelry, decorative purposes, electrical contacts, thermocouples, crucibles, electrodes, hypodermic needles, and medical accessories. Iridium enhances resistance of platinum to chemical attack and corrosion. It also enhances hardness and tensile strength. The radioisotope Ir-192 is used in examination of ferrous welds and in other radiographic applications.
Reactions
At ordinary temperatures iridium exhibits strong resistance to chemical attack. At elevated temperatures of about 600°C, iridium metal combines with oxygen to form a coating of iridium dioxide, IrO2. Similarly, the metal reacts with halogens only at elevated temperatures. It reacts with fluorine at 250°C, forming iridium hexafluoride, IrF6, and, to a lesser extent, iridium tetrachloride IrCl4. Heating with chlorine at 600°C produces iridium trichloride, IrCl3. Iridium forms alloys with several metals—mostly platinum group metals.
Iridium does not react with concentrated acids or with molten alkalies.
Chemical Properties
Black Powder
Physical properties
Iridium is a hard, brittle, white, metallic substance that is almost impossible to machine.It is neither ductile nor malleable. Iridium will only oxidize at high temperatures and is themost corrosive-resistant metal known. This is why it was used to make the standard meter barthat is an alloy of 90% platinum and 10% iridium.
At about the time of the French Revolution, it was decided to determine the length ofthe meter bar by first calculating the distance from the North Pole to the equator runningthrough Paris. This distance was then divided into equal lengths of 1/10,000,000. A singleunit of this distance was then called a “meter” (“measure” in Greek). This platinum-iridiummeter bar, currently preserved in France, was for many years the standard unit of lengthin the metric system that is based on the decimal system. However, this metal bar is nolonger used as the standard meter. Instead, the meter is now defined by scientists in termsof the length of the path traveled by light in a vacuum at the time of 1/299,792,458 of asecond.
Iridium is highly resistant to attack by other chemicals and is one of the most dense elementsfound on Earth. Its melting point is 2,410°C, its boiling point is 4,130°C, and itsdensity is 22.560 g/cm
3.
Isotopes
There are 55 isotopes of iridium, two of which are stable and account for theelement’s total existence on Earth. Those two are Ir-191, which makes up 37.3% of theamount in the Earth’s crust, and Ir-193, which constitutes 62.7% of iridium’s existenceon Earth. All the other 53 isotopes of iridium are radioactive with half-lives ranging froma few microseconds to a few hours or days and up to a few hundred years. Theseunstable isotopes are all artificially produced.
Origin of Name
The name iridium comes from the Latin word iris, meaning “rainbow,”
because of the element’s highly colored salts.
Occurrence
Iridium is the 83rd most abundant element and is found mixed with platinum, osmium,and nickel ores. The minerals containing iridium are found in Russia, South Africa, Canada,and Alaska.
Iridium metal is separated from its other metal ores when the combined minerals aredissolved with a strong acid know as aqua regia, which is a mixture of 25% nitric acid and75% hydrochloric acid. Aqua regia is the only acid that will dissolve platinum and gold.Once the platinum and other metals are dissolved, the iridium, which is insoluble in thisstrong acid, becomes the residue. The refined iridium ends up in the form of either powderor crystals.
An interesting story as to how most of the iridium appeared on Earth was explainedrecently by scientists who discovered a thin layer of iridium in the sediments that were laiddown in the Earth’s crust at the end of the Cretaceous period. This was a period about 65 millionyears ago when meteors and asteroids crashed into the Earth. These extraterrestrial bodiescontained a high percentage of iridium. Dust from the impact spread around the Earth andblocked the sun for months, resulting in the extinction of many plants and animals, includingthe dinosaurs. This extensive dust cloud also deposited a thin coat of the element iridium thatwas contained in the fiery bolides.
Characteristics
Iridium is one of the so-called platinum group of 6 transition elements (Ru, Rh, and Pd ofperiod 5 and Os, Ir, and Pt of period 6). It is resistant to strong acids, including aqua regia.It is the only metal that can be used in equipment that must withstand temperatures up to2,300°C or 4,170°F. Iridium can be poured into casts after it becomes molten. As it cools, itbecomes crystalline and, while in this state, can be pulled into wires and formed into sheets.Unlike steel, which becomes more malleable (less brittle) after annealing (a process of heatingfollowed by slowly cooling), iridium is just the opposite—it becomes more brittle and impossibleto work into shapes after cooling.
History
Discovered in 1803 by Tennant in the residue left when crude platinum is dissolved by aqua regia. The name iridium is appropriate, for its salts are highly colored. Iridium, a metal of the platinum family, is white, similar to platinum, but with a slight yellowish cast. It is very hard and brittle, making it very hard to machine, form, or work. It is the most corrosion-resistant metal known, and was used in making the standard meterbar of Paris, which is a 90% platinum–10% iridium alloy. This meter bar was replaced in 1960 as a fundamental unit of length (see under Krypton). Iridium is not attacked by any of the acids nor by aqua regia, but is attacked by molten salts, such as NaCl and NaCN. Iridium occurs uncombined in nature with platinum and other metals of this family in alluvial deposits. It is recovered as a by-product from the nickel mining industry. The largest reserves and production of the platinum group of metals, which includes iridium, is in South Africa, followed by Russia and Canada. The U.S. has only one active mine, located at Nye, MT. The presence of iridium has recently been used in examining the Cretaceous-Tertiary (K-T) boundary. Meteorites contain small amounts of iridium. Because iridium is found widely distributed at the K-T boundary, it has been suggested that a large meteorite or asteroid collided with the Earth, killing the dinosaurs, and creating a large dust cloud and crater. Searches for such a crater point to one in the Yucatan, known as Chicxulub. Iridium has found use in making crucibles and apparatus for use at high temperatures. It is also used for electrical contacts. Its principal use is as a hardening agent for platinum. With osmium, it forms an alloy that is used for tipping pens and compass bearings. The specific gravity of iridium is only very slightly lower than that of osmium, which has been generally credited as being the heaviest known element. Calculations of the densities of iridium and osmium from the space lattices give values of 22.65 and 22.61 g/cm3, respectively. These values may be more reliable than actual physical measurements. At present, therefore, we know that either iridium or osmium is the densest known element, but the data do not yet allow selection between the two. Natural iridium contains two stable isotopes. Forty-five other isotopes, all radioactive, are now recognized. Iridium (99.9%) costs about $100/g.
Uses
In manufacturing crucibles; in hardening platinum; in making nibs for fountain-pen points.
Uses
Iridium’s most common use is as an alloy metal that, when added to platinum, makes itharder and more durable. It is also mixed with other metals to make electrical contacts, thermocouples(two dissimilar metals joined to form a special type of thermometer), and instrumentsthat will withstand high temperatures without breaking down. It is also used to makespecial laboratory vessels because iridium will not react with most chemical substances. Analloy of iridium and platinum is used as the standard kilogram weight because it is noncorrosiveand will not oxidize and, thus, change its weight over long periods of time.
The radioisotope, iridium-192, is used to treat cancer and to take X-ray pictures of metalcastings to detect flaws. Iridium is also used as a catalyst for several chemical reactions.
Uses
The primary use of iridium is as a hardening agent in platinum alloys. Other uses are for the making of crucibles and devices that require high temperatures. Osmium/iridium alloys are used for compass bearings. Iridium is commonly used in complexes like Ir(mppy)3 and other complexes in polymer LED technology to increase the efficiency from 25% to about 100% due to triplet harvesting. Used in high-dose radiation therapy for the treatment of prostate and other forms of cancer. Iridium is used in tips of ballpoint pens. Iridium is used as a catalyst for carbonylation of methanol to create acetic acid. At one time, iridium as an alloy with platinum, was used in bushing the vents of heavy ordnance and, in a finely powdered condition (iridium black) for painting porcelain black.
Definition
A white transition
metal that is highly resistant to corrosion.
It is used in electrical contacts, in spark
plugs, and in jewelry.
Symbol: Ir; m.p. 2410°C; b.p. 4130°C;
r.d. 22.56 (17°C); p.n. 77; r.a.m. 192.217.
Definition
Metallic element of atomic number 77, one of the
platinum metals, group VIII of the periodic table,
aw 192.22, valences = 1,2,3,4,6, two stable isotopes.
Definition
iridium: Symbol Ir. A silvery metallictransition element; a.n. 77; r.a.m. 192.20;r.d. 22.42; m.p. 2410°C; b.p. 4130°C.It occurs with platinum and ismainly used in alloys with platinumand osmium. The element forms arange of iridium(III) and iridium(IV)complexes. It was discovered in 1804by Smithson Tennant (1761–1815).
Hazard
The elemental metal form of iridium is almost completely inert and does not oxidizeat room temperatures. But, as with several of the other metals in the platinum group,several of iridium’s compounds are toxic. The dust and powder should not be inhaled oringested.
Flammability and Explosibility
Non flammable
Industrial uses
Iridium (symbol Ir) is a grayish-white metal ofextreme hardness. It is insoluble in all acids andin aqua regia. The melting point is 2447°C, andthe specific gravity is 22.50. It occurs naturallywith the metal osmium as an alloy, known asosmiridium, 30 to 60% osmium, used chieflyfor making fountain-pen points and instrumentpivots.
Iridium is employed as a hardener for platinum,the jewelry alloys usually containing10%. With 35% iridium the tensile strength ofplatinum is increased to 965 MPa. Iridium wireis used in spark plugs because it resists attackof leaded aviation fuels.
Because of its scarcity and high cost, applications of iridium are severely limited. Although iridium metal and many of its complex compounds are good catalysts, no large-scale commercial application for these has been developed. In general, other platinum metals have superior catalytic properties. Platinum-iridium alloys are used for electrodes in spark plugs that are unusually resistant to fouling by antiknock lead additives. Iridium rhodium thermocouples are used for high-temperature applications, where they have unique stability.
Synthesis
Iridium is a by-product of the nickel and copper mining and refining processes. During the electrolytic refining of nickel and copper, precious metals such as gold and silver, platinum-based elements, and non-metallic elements such as selenium and tellurium accumulate on the positive electrode. This sludge has to go into solution in order to separate the metals from it. The exact method depends on the composition of the mixture, but there are two main types: dissolved in aqua regia after adding sodium peroxide, or dissolved directly in a mixture of chlorine and hydrochloric acid.
Purification Methods
Iridium is a silver white hard solid which oxidises on the surface in air. Scrape the outer tarnished layer until silver clear and store it under paraffin. It is stable to acids but dissolves in aqua regia. [Gilchrist Chem Rev 32 277 1943.]
Structure and conformation
The space lattice of Ir belongs to the cubic system, and its face-centered cubic lattice has a lattice
constant of a=0.38312 nm and Ir–Ir=0.2709 nm (18℃).