Description
Etofenprox belongs to a pyrethroid derivative that is commonly used as an insecticide in agriculture, horticulture, viticulture, forestry, animal health and public health against various insect pests. It can be found as an active ingredient in a variety of pest control products, which is effective to control broad spectrum of pests, such as crawling and flying insects. In the field of agriculture, etofenprox can be applied on a broad range of crops, including rice, fruits, vegetables, corn, soybeans and tea, which is poorly absorbed by roots and little translocation occurs within plants. It is also used for the public health by controlling adult mosquitoes, non biting midges, biting and non-biting flies, which may carry the pathogen. Besides, etofenprox can be found as an component in flea and tick medications for dogs and cats.
Etofenprox is a new type of insecticide with low mammalian toxicity, which can be decomposed in soil by anaerobic and aerobic microorganisms. It functions by strongly disrupting the transmission of nervous impulses and causes paralysis and death to the target insect.
Chemical Properties
solid
Uses
Etofenprox is used to control a wide variety of insects on fruit, tea,
soyabeans and many vegetables. It is also used in public health and animal
health.
Definition
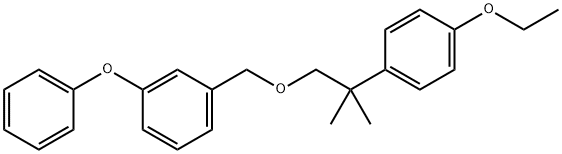
ChEBI: An aromatic ether that is the 3-phenoxybenzyl ether of 2-(4-ethoxyphenyl)-2-methylpropan-1-ol.
Agricultural Uses
Insecticide: Approved for use in the U.S. and more than a dozen
EU countries.
Trade name
MTI 500®; PUNKASO®; TREBON®;
ZOECON® RF-316
Metabolic pathway
Etofenprox is one of the few members of a new class, the non-ester
pyrethroids. It lacks the ester bond and therefore is not subject to facile
chemical or biochemical hydrolysis. Metabolic options will be limited to
oxidative processes. Little has been published in the scientific literature.
Its photochemistry has been described and metabolic oxidation products
have been noted.
Degradation
Etofenprox is stable in acid and alkaline media for more than 100 days at
80 °C. It is reasonably stable to light under normal conditions of use but it
is photodegradable. Under conditions where allethrin was degraded with
a half-life of 0.5 hours, etofenprox had a half-life of 3 hours (Tsao and Eto,
1990).
The major product was the ester formed by oxidation at the benzylic
carbon atom to form 2-(4-ethoxyphenyl)-2-methylpropyl 3-phenoxybenzoate
(2), clearly the result of hydroxylation and dehydrogenation.
This ester was found to be non-toxic to houseflies. It was cleaved to form 3PBA (5) and the alcohol (6). 6 was degraded via decarboxylation to 7 and
8. A concurrent reaction of etofenprox was O-de-ethylation to the phenol
(3). A similar array of products was seen in both aqueous suspension and
as a thin film on glass. It should be noted that this study did not utilise
radiolabelled compound.