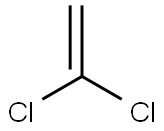
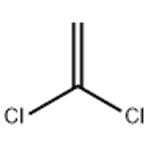
Chemical Properties
Colorless liquid. Readily polymerizes. Insoluble in water.
Commercial product contains small proportion of
inhibitor.
Chemical Properties
Vinylidene chloride is a volatile liquid.
Mild, sweet odor resembling chloroform. The odor threshold
in air is 500 ppm.
Physical properties
Colorless liquid or gas with a mild, sweet, chloroform-like odor. The average least detectable odor
threshold concentration in water at 60 °C and in air at 40 °C was 1.6 mg/L (Alexander et al.,
1982).
Uses
VDC is used to make various kinds of chemical intermediates,
agricultural chemicals, SARAN?polyvinylidene chloride
(PVDC) resins and films, PVDC latex coatings, and
photographic and X-ray films.
Uses
1,1-Dichloroethylene (1,1-DCE) is used toproduce vinylidene copolymers for films andcoatings.
Uses
Intermediate in the production of "vinylidene polymer plastics" such as Saran (Dow) .
Production Methods
VDC is prepared commercially by the dehydrochlorination
of 1,1,2-trichloroethane using a slight excess of lime or
caustic as shown in the reaction schematic. About
200 ppm of monomethyl ether of hydroquinone (MEHQ) is
added to prevent polymer formation and preserve product
quality.
Definition
ChEBI: A member of the class of chloroethenes that is ethene in which both of the hydrogens attached to one of the carbons are replaced by chlorines.
General Description
A clear colorless liquid with a chloroform-like odor. Flash point 0°F. Boiling point 99°F. Denser (at 10.1 lb / gal) than water and insoluble in water. Hence sinks in water. May polymerize exothermically if heated or contaminated. If the polymerization takes place inside a container, the container may rupture violently. Vapors heavier than air.
Air & Water Reactions
Highly flammable. Insoluble in water.
Reactivity Profile
Peroxidizable monomer, such as VINYLIDENE CHLORIDE, may initiate exothermic polymerization of the bulk material [Bretherick 1979. p. 160, 187]. Mixing vinylidene chloride in equal molar portions in a closed container with any of the following substances caused the temperature and pressure to increase: chlorosulfonic acid, nitric acid, or oleum [NFPA 1991]. It's reaction products with ozone are particularly dangerous, [Dow Chemical, 1968]. This may extend to other powerful oxidants, as various peroxides are produced.
Health Hazard
Vapor can cause dizziness and drunkenness; high levels cause anesthesia. Liquid irritates eyes and skin.
Health Hazard
1,1-DCE exhibits low acute toxicity. Vaporsare irritant to the mucous membranes. Athigh concentrations it produces narcoticeffects. Chronic exposure to a 50-ppm con centration for 8 hours/day, 5 days/week for6 months resulted in liver and kidney injuryin experimental animals. The liquid in con tact with the eyes causes irritation. The LC50value in rats is within the range 6300 ppm fora 4-hour exposure period. The oral toxicity islow. A lethal dose by subcutaneous admin istration is 3700 mg/kg in rabbits. Ingestioncan cause nausea and vomiting.
Tests on laboratory animals indicate that1,1-DCE is cancer causing. Rats and micesubjected to 12 months’ inhalation of thiscompound developed tumors of the liver,kidney, skin, and blood. Carcinogenicity inhumans is not reported.
Fire Hazard
Flammable liquid; flash point (closed cup) -18°C(0°F) (flash point data reported in the
literature differ); vapor pressure 500 torr at
20°C (68°F); vapor density 3.34 (air=1);
the vapor is heavier than air and can travel
a considerable distance to a source of igni tion and flash back; autoignition temperature 570°C (1058°F); fire-extinguishing agent: dry
chemical, CO2, or foam; use water to keep
fire-exposed containers cool and to flush any
spill.
1,1-DCE vapors form explosive mixtures
with air within the range 7.3–16.0% by
volume in air. It polymerizes at elevated
temperatures. If polymerization occurs in
a closed container, the container may rup ture violently. Polymerization is inhibited in
the presence of 200 ppm of hydroquinone
monomethyl ether (Aldrich 1997). It forms
a white deposit of peroxide on long stand ing which may explode. It decomposes when
involved in fire, producing toxic hydrogen
chloride. Reactions with concentrated min eral acids are exothermic.
Safety Profile
Suspected carcinogen
with experimental carcinogenic,
neoplastigenic, tumorigenic, and teratogenic
data. Poison by inhalation, ingestion, and
intravenous routes. Moderately toxic by
subcutaneous route. Human systemic effects
by inhalation: general anesthesia, liver and
hdney changes. Experimental reproductive
effects. Mutation data reported. See also
VINYL CHLORIDE. A very dangerous fire
hazard when exposed to heat or flame.
Moderately explosive in the form of gas
when exposed to heat or flame. It forms
explosive peroxides upon exposure to air.
Potentially explosive reaction with
chlorotrifluoroethylene at 18O℃. Reaction
with ozone forms dangerous products.
Explosive reaction with perchloryl fluoride
when heated above 100℃. Also can explode
spontaneously. Reacts violently with
chlorosulfonic acid, HNO3, oleum. Can
react vigorously with oxidizing materials. To
fight fire, use alcohol foam, CO2, dry
chemical. When heated to decomposition it
emits toxic fumes of Cl-. See also
CHLORINATED HYDROCARBONS,
ALIPHATIC.
Potential Exposure
Vinylidene chloride is used in the
manufacture of 1,1,1-trichloroethane (methyl chloroform).
However, the manufacture of polyvinylidene copolymers is
the major use of VDC. The extruded films of the copolymers
are used in packaging and have excellent resistance to
water vapor and most gases. The chief copolymer is Saran
(polyvinylidene chloride/vinyl chloride), a transparent film
used for food packaging. The films shrink when exposed to
higher than normal temperatures. This characteristic is
advantageous in the heat-shrinking of overwraps on packaged
goods and in the sealing of the wraps. Applications of
VDC latexes include mixing in cement to produce highstrength
mortars and concretes, and as binders for paints
and nonwoven fabrics providing both water resistance and
nonflammability. VDC polymer lacquers are also used in
coating films and paper. VDC is also used to produce
fibers. Monofilaments, made by extruding the copolymer,
are used in the textile industry as furniture and automobile
upholstery; drapery fabric; outdoor furniture; venetian-blind
tape; and filter cloths.
Carcinogenicity
The IARC has concluded that
there is inadequate evidence in humans and limited evidence
in experimental animals for the carcinogenicity of VDC and
has placed it in its Group 3 category as not classifiable as to its
carcinogenicity to humans.
This conclusion is consistent with the evaluation by the
EPA, where VDC exhibits suggestive evidence of carcinogenicity
but not sufficient evidence to assess human carcinogenic
potential following inhalation exposure in studies in
rodents.
Environmental Fate
Biological. 1,1-Dichloroethylene significantly degraded with rapid adaptation in a static-culture
flask-screening test (settled domestic wastewater inoculum) conducted at 25 °C. Complete
degradation was observed after 14 d. At concentrations of 5 and 10 mg/L, the amount lost due to
volatilization at the end of 10 d was 24 and 15%, respectively (Tabak et al., 1981).
Soil. In a methanogenic aquifer material, 1,1-dichloroethylene biodegraded to vinyl chloride
(Wilson et al., 1986). Under anoxic conditions, indigenous microbes in uncontaminated sediments
degraded 1,1-dichloroethylene to vinyl chloride (Barrio-Lage et al., 1986).
Photolytic. Photooxidation of 1,1-dichloroethylene in the presence of nitrogen dioxide and air
yielded phosgene, chloroacetyl chloride, formic acid, HCl, carbon monoxide, formaldehyde, and
ozone (Gay et al., 1976). At 298 K, 1,1-dichloroethylene reacts with ozone at a rate of 3.7 x 10
-21
cm
3/molecule?sec (Hull et al., 1973).
Chemical/Physical. At temperatures exceeding 0 °C in the presence of oxygen or other
catalysts, 1,1-dichloroethylene will polymerize to a plastic (Windholz et al., 1983). The alkaline
hydrolysis of 1,1-dichloroethylene yielded chloroacetylene. The reported hydrolysis half-life at 25
°C and pH 7 is 1.2 x 108 yr (Jeffers et al., 1989).
Shipping
UN1303 Vinylidene chloride, stabilized, Hazard
Class: 3; Labels: 3-Flammable liquid.
Incompatibilities
Readily forms explosive peroxides; violent
polymerization from heat or on contact with oxidizers,
chlorosulfonic acid; nitric acid; or oleum; or under the
influence of oxygen, sunlight, alkali metals; aluminum,
copper. Explosive on heating or on contact with flames.
Inhibitors, such as the monomethyl ether of hydroquinone
are added to prevent polymerization.
Toxics Screening Level
The initial threshold screening level (ITSL) is 7.3 μg/m3 (annual averaging time). The initial
risk screening level (IRSL) for 1,1’-dichloroethylene is 0.15 μg/m3 (annual averaging time)
and the secondary screening level (SRSL) is 1.5 μg/m3 (annual averaging time).
Waste Disposal
Return refillable compressed
gas cylinders to supplier. Consult with environmental regulatory
agencies for guidance on acceptable disposal practices.
Generators of waste containing this contaminant
(≥100 kg/mo) must conform to EPA regulations governing
storage, transportation, treatment, and waste disposal.
Incineration, preferably after mixing with another combustible
fuel. Care must be exercised to assure complete combustion
to prevent the formation of phosgene. An acid scrubber
is necessary to remove the halo acids produced.