Chemical Properties
colourless liquid
Chemical Properties
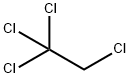
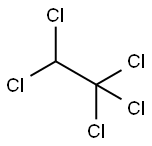
1,1,1,2-Tetrachloroethane is a colorless to yellowish-red liquid.
Uses
1,?1,?1,?2-?Tetrachloroethane is a chlorinated hydrocarbon and used as a solvent for cellulose, acetate, fat, waxes, greases, rubber, and sulfur. It is a contaminant in groundwater. 1,?1,?1,?2-?Tetrachloroethane can also induce heptotoxicity.Environmental contaminants; food contaminants.
Definition
ChEBI: 1,1,1,2-tetrachloroethane is a member of chloroethanes.
General Description
Clear colorless liquid.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
1,1,1,2-TETRACHLOROETHANE is incompatible with strong oxidizing agents and strong bases. 1,1,1,2-TETRACHLOROETHANE is also incompatible with dinitrogen tetraoxide, 2,4-dinitrophenyl disulfide, potassium, potassium hydroxide, nitrogen tetraoxide, sodium and sodium potassium alloy. 1,1,1,2-TETRACHLOROETHANE may react with chemically active metals, strong caustics, hot iron, aluminum and zinc in presence of steam. 1,1,1,2-TETRACHLOROETHANE may also react with mixtures of dinitrogen tetraoxide with halocarbons.
Fire Hazard
Literature sources indicate that 1,1,1,2-TETRACHLOROETHANE is nonflammable.
Safety Profile
A skin and severe eye irritant. Questionable carcinogen with experimental carcinogenic data. Incompatible with dinitrogen tetraoxide, 2,4-dinitrophenyl disulfide, potassium, potassium hydroxide, nitrogen tetroxide, sohum, sodium potassium alloy. Mutation data reported. When heated to decomposition it emits very toxic fumes of Cl-.
Potential Exposure
1,1,1,2-Tetrachloroethane is used as a solvent and in manufacture of insecticides, herbicides, soilfumigants; blanches, paints, and a number of widely used products; as are the other chloroethanes.
Shipping
UN1702 Tetrachloroethane, Hazard Class: 6.1; Labels: 6.1-Poisonous materials.
Incompatibilities
1,1,1,2-Tetrachloroethane is incompatible with strong oxidizing agents and strong bases. It is also incompatible with dinitrogen tetraoxide, 2,4-dinitrophenyl disulfide, potassium, potassium hydroxide, nitrogen tetraoxide, sodium and sodium potassium alloy. It may react with chemically active metals, strong caustics, hot iron, aluminum and zinc in presence of steam. It may also react with mixtures of dinitrogen tetraoxide with halocarbons
.
Toxics Screening Level
The Initial Risk Screening Level (IRSL) for 1,1,1,2-tetrachloroethane is 0.1 μg/m3 based on an annual averaging time.
Waste Disposal
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal.