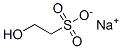
Sodium isethionate
- Product NameSodium isethionate
- CAS1562-00-1
- CBNumberCB8253893
- MFC2H5NaO4S
- MW148.11
- EINECS216-343-6
- MDL NumberMFCD00007534
- MOL File1562-00-1.mol
- MSDS FileSDS
Chemical Properties
| Melting point | 191-194 °C(lit.) |
| Density | 1762.7[at 20℃] |
| bulk density | 800kg/m3 |
| storage temp. | Store below +30°C. |
| solubility | H2O: 0.1 g/mL, clear, colorless |
| form | Fine Powder |
| color | White |
| PH | 7.0-11.0 (20g/l, H2O, 20℃) |
| Water Solubility | SOLUBLE |
| BRN | 3633992 |
| Stability | Stable. Hygroscopic. Incompatible with strong oxidizing agents, strong acids. |
| Cosmetics Ingredients Functions | CLEANSING ANTISTATIC HAIR CONDITIONING SKIN CONDITIONING |
| InChI | InChI=1S/C2H6O4S.Na/c3-1-2-7(4,5)6;/h3H,1-2H2,(H,4,5,6);/q;+1/p-1 |
| InChIKey | LADXKQRVAFSPTR-UHFFFAOYSA-M |
| SMILES | [Na+].[O-]S(CCO)(=O)=O |
| LogP | -4.6 at 20℃ |
| CAS DataBase Reference | 1562-00-1(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
Safety
| Symbol(GHS) |

|
|||||||||
| Signal word | Warning | |||||||||
| Hazard statements | H315-H319 | |||||||||
| Precautionary statements | P264-P280-P302+P352+P332+P313+P362+P364-P305+P351+P338+P337+P313 | |||||||||
| Hazard Codes | Xn | |||||||||
| Risk Statements | 40 | |||||||||
| Safety Statements | 24/25-36-22 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | KI7700000 | |||||||||
| TSCA | TSCA listed | |||||||||
| HS Code | 29055910 | |||||||||
| Storage Class | 13 - Non Combustible Solids | |||||||||
| Hazardous Substances Data | 1562-00-1(Hazardous Substances Data) | |||||||||
| Toxicity | LD50 orally in Rabbit: > 2500 mg/kg | |||||||||
| NFPA 704: |
|