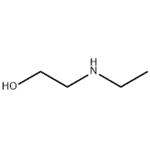
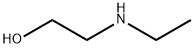Chemical Properties
Clear colorless to slightly yellowish liquid
Uses
Solvent, intermediate.
Uses
2-(Ethylamino)ethanol is an efficient compound used for inhibiting corrosion. This high activity of the inhibitor may be interpreted both by its ability to stabilize the hydroxide surface film and by its adsorption onto bare sites of the metal.
General Description
A colorless liquid with an amine-like odor. Flash point 160°F. Slightly less dense than water. May mildly irritate skin and eyes. At high temperature may decompose to yield toxic oxides of nitrogen.
Air & Water Reactions
Soluble in water.
Reactivity Profile
ETHYLAMINOETHANOL is an aminoalcohol. Amines are chemical bases. They neutralize acids to form salts plus water. These acid-base reactions are exothermic. The amount of heat that is evolved per mole of amine in a neutralization is largely independent of the strength of the amine as a base. Amines may be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated by amines in combination with strong reducing agents, such as hydrides.
Health Hazard
Inhalation or contact with material may irritate or burn skin and eyes. Fire may produce irritating, corrosive and/or toxic gases. Vapors may cause dizziness or suffocation. Runoff from fire control or dilution water may cause pollution.
Flammability and Explosibility
Non flammable
Safety Profile
Poison by skin contact.
Moderately toxic by ingestion and
intraperitoneal routes. A skin and severe eye
irritant. Flammable when exposed to heat or flame; can react vigorously with oxidizers.
To fight fre, use alcohol foam, dry
chemical, CO2. When heated to
decomposition it emits toxic fumes of NOx.
Toxics Screening Level
The initial threshold screening level (ITSL) for 2-ethylaminoethanol is 1 μg/m3 based on an annual averaging time.