Description
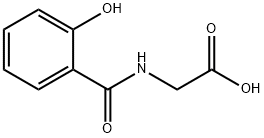
Salicyluric acid is an N-acylglycine in which the acyl group is
specified as 2-hydroxybenzoyl. It has a role as a uremic toxin and a
human xenobiotic metabolite. It is a N-acylglycine and a secondary
carboxamide. It is functionally related to a glycine. It is a conjugate acid of a salicylurate.
Chemical Properties
Light gray crystalline powder
Uses
Metabolite of Salicylic Acid (S088125).
2-Hydroxyhippuric acid is a phenol marker. Can be associated with dietary intake or bacterial overgrowth.
Indicators of Detoxification: A conjugate of the amino acid glycine and hydroxybenzoic acid (salicylic acid). Intake of aspirin (salicylates) or the growth of salicylate-producing gastrointestinal bacteria may elevate levels. Also increased after the ingestion of the artificial sweetener aspartame (Nutrasweet).
Definition
ChEBI: An N-acylglycine in which the acyl group is specified as 2-hydroxybenzoyl.
Synthesis
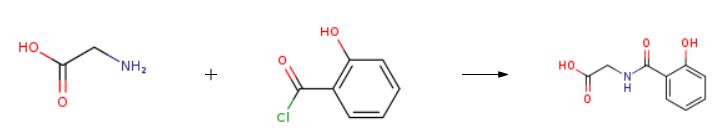
The synthesis of Salicyluric acid is as follows:
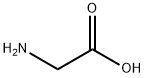
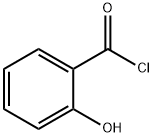
To a solution of 5 g of glycine in 50ml of 10%NaOH was stirred at room temperature upon slowaddition of salicyloyl chloride, and the resultingmixture was stirred overnight. Progress of the reaction was followed by TLC (hexane : ethylacetate, 7 : 3).Then the reaction mixture was treated with crushed iceupon stirring. Concentrated HCl was added dropwiseand stirring was continued until the mixture turnedacidic. For removing the residual benzoic acid the mixture was treated with 10-12 mL of CCl4, boiled and then filtered. A pinkish white solid was obtained, yield 90%, mp 165°C.