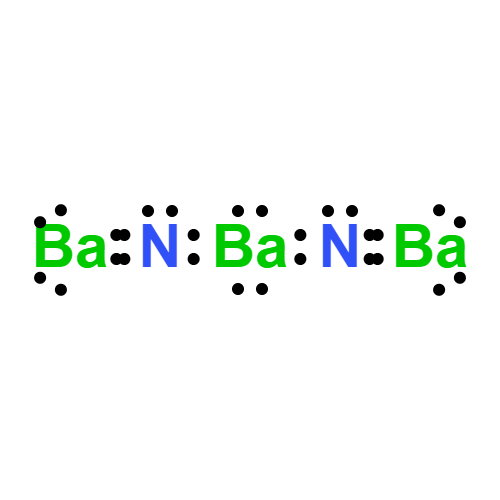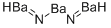
BARIUM NITRIDE
- Product NameBARIUM NITRIDE
- CAS12047-79-9
- CBNumberCB8170031
-
MFBa3N2
Lewis structure
- MW439.99
- EINECS234-977-1
- MDL NumberMFCD00049718
- MOL File12047-79-9.mol
- MSDS FileSDS
Chemical Properties
| Boiling point | 1000, vacuum [ALF93] |
| Density | 4.780 |
| solubility | reacts with H2O |
| form | Powder |
| color | black |
| Water Solubility | decomposed in H2O [CRC10] |
| EWG's Food Scores | 1 |
| EPA Substance Registry System | Barium nitride (Ba3N2) (12047-79-9) |
| UNSPSC Code | 12352300 |
| NACRES | NA.23 |
Safety
| Symbol(GHS) |

|
| Signal word | Warning |
| Hazard statements | H302+H332 |
| Precautionary statements | P261-P264-P270-P271-P301+P312-P304+P340+P312 |
| Hazard Codes | Xn |
| Risk Statements | 20/22 |
| Safety Statements | 28 |
| RIDADR | UN 2813 4.3 / PGI |
| WGK Germany | 3 |
| Toxicity | LD50 orl-mus: 46,100 mg/kg TOIZAG 22,119,75 |