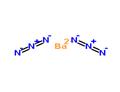
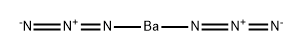Description
Barium azide, is a crystalline solid with not less than 50% water by mass that explodes when shocked or heated. Barium azide decomposes and gives off nitrogen at 240°F (115.5°C) and is soluble in water. The four-digit UN identification number for barium azide is 1571. Its primary use is in high explosives.
Chemical Properties
Barium azide is a flammable, crystalline solid
which can be used or transported in solution.
Physical properties
Colorless monoclinic crystal; density 2.936 g/cm
3; decomposes at 120°C; soluble in water, slightly soluble in ethanol.
Preparation
Barium azide may be prepared by reacting sodium azide with a soluble barium salt. The solution is concentrated to allow crystals grow. Crystals will explode if fully dried, or subject to friction. Product should be stored damp with ethanol.
General Description
A slurry of white crystals. When dry, a high explosive that easily ignited and quick to burn vigorously. Wetting reduces sensitivity to shock and heat. Generates toxic oxides of nitrogen when burned.
Air & Water Reactions
Highly flammable. Soluble in water.
Reactivity Profile
BARIUM AZIDE is a suspension or slurry of of an unstable solid in sufficient water to allow safe shipping and handling. May explode if allowed to dry out and then heated or shocked. Incompatible with strong oxidizing agents, strong reducing agents.
Hazard
Explodes when shocked or heated.
Health Hazard
Some are toxic and may be fatal if inhaled, swallowed or absorbed through skin. Contact may cause burns to skin and eyes. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may cause pollution.
Safety Profile
A poison. Moderate
explosion hazard when shocked or heated to
275'. Spontaneously flammable in air. Very
unstable. When heated to decomposition it
emits toxic fumes of NO,. See also BARIUM COWOUNDS (soluble) and
AZIDES.
Potential Exposure
Barium Azide is used in high
explosives.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts the skin,remove contaminated clothing and wash immediately withsoap and water. Seek medical attention immediately. If thischemical has been inhaled, remove from exposure, begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR if heart actionhas stopped. Transfer promptly to a medical facility. Whenthis chemical has been swallowed, get medical attention.Give large quantities of water and induce vomiting. Do notmake an unconscious person vomit. If weakness or faintingis present, lay the person down flat with feet elevated. Seealso First Aid section in “Barium” entry.
storage
Color Code—Red Stripe: Flammability Hazard:Do not store in the same area as other flammable materials.Barium azide must be stored to avoid contact with carbondisulfide since violent reactions occur. Store in tightlyclosed containers in a cool, well-ventilated area from anything which could disturb or shock barium azide. Sourcesof ignition, such as smoking and open flames are prohibitedwhere barium azide is handled, used, or stored. Keepingbarium azide wet greatly reduces its fire and explosion hazard. Wherever barium azide is used, handled, manufactured,or stored, use explosion-proof electrical equipment andfittings.
Shipping
UN1571 Barium azide wetted with not ,50%
water, by mass, Hazard Class: 4.1; Labels: 4.1—Flammable
solid, 6.1—Poisonous materials. UN0224 Barium Azide dry
or wetted with ,50% water, by mass, Hazard Class: 1.1A;
Labels: 1.1A—Explosive (with a mass explosion hazard);
A—Substances which are expected to mass detonate very
soon after fire reaches them. Packing Group 1.
Incompatibilities
Carbon disulfide. It can explode when
heated or shocked.