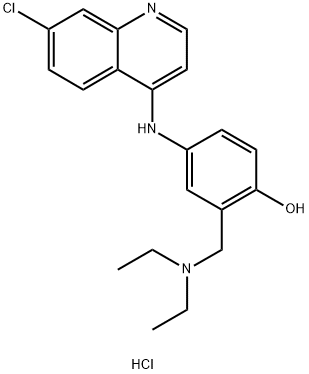
Chemical Properties
Yellow crystalline solid; odorless; bitter. Soluble in water; sparingly soluble in alcohol; very slightly soluble in benzene, chloroform, and ether.
Originator
Camoquin HCl,Parke Davis,US,1950
Manufacturing Process
72.8 g (0.5 mol) of p-aminophenol hydrochloride is dissolved in 500 cc of
water and added to 99 g (0.5 mol) of 4,7-dichloroquinoline. After a few
minutes of warming in a steam bath, 4-(4'-hydroxyanilino)-7-chloroquinoline
hydrochloride, of sufficient purity for use in further experiments, precipitates
as a yellow crystalline solid. Recrystallized from methanol, the MP is over
300°C.
A mixture consisting of 13.5 g of 4-(4'-hydroxyanilino)-7-chloroquinoline
hydrochloride dissolved in absolute ethanol is treated with a solution of 4.38 g of diethylamine and 1.8 g of paraformaldehyde in 20 cc of absolute ethanol.
The reaction mixture is heated under reflux for 16 hours, evaporated to onehalf
volume and the warm solution treated with an excess of hydrogen
chloride dissolved in absolute ethanol. Acetone is added to the warm solution
until it becomes turbid and then the solution is cooled. The crude
dihydrochloride which separates is collected and purified by recrystallization
from methanol; MP 240-242°C.
By using an equivalent amount of 4-(4'-hydroxyanilino)-7-bromoquinoline in
the above procedure, 4-(3'-diethylaminomethyl-4'-hydroxyanilino)-7-
bromoquinoline dihydrochloride is obtained; MP (base) 206-208°C dec.