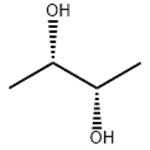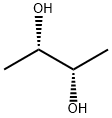
(S,S)-2,3-Butanediol
- Product Name(S,S)-2,3-Butanediol
- CAS19132-06-0
- CBNumberCB8117911
- MFC4H10O2
- MW90.12
- MDL NumberMFCD00063648
- MOL File19132-06-0.mol
- MSDS FileSDS
Chemical Properties
| Melting point | 20°C |
| Boiling point | 179-182 °C (lit.) |
| alpha | 13 º (c=neat) |
| Density | 0.987 g/mL at 25 °C (lit.) |
| refractive index | n |
| Flash point | 185 °F |
| storage temp. | 2-8°C |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| pka | 14.67±0.20(Predicted) |
| form | Oily Liquid |
| color | Slightly yellow |
| Specific Gravity | 0.987 |
| PH | 9-10 (500g/l, H2O, 20℃) |
| explosive limit | 3.1-11.4%(V) |
| optical activity | [α]20/D +13°, neat |
| Water Solubility | soluble |
| BRN | 1718899 |
| Stability | hygroscopic |
Safety
| Symbol(GHS) |

|
|||||||||
| Signal word | Warning | |||||||||
| Hazard statements | H227 | |||||||||
| Precautionary statements | P210-P280-P370+P378-P403+P235-P501 | |||||||||
| Hazard Codes | Xi | |||||||||
| Safety Statements | 24/25 | |||||||||
| RIDADR | NA 1993 / PGIII | |||||||||
| WGK Germany | 3 | |||||||||
| HS Code | 29053995 | |||||||||
| NFPA 704: |
|