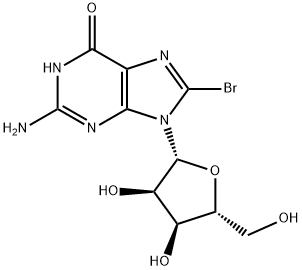
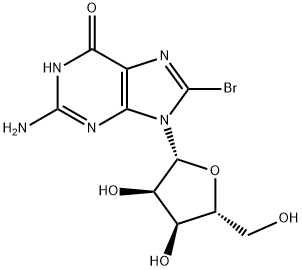
Uses
8-Bromoguanosine-13C2,15N is an intermediate in the synthesis of 8-Aminoguanosine-13C2,15N (A609877). 8-Aminoguanosine-13C2,15N is an isotopic labelled compound of 8-Aminoguanosine (A609875), which is a potent inhibitor of purine nucleoside phosphorylase.
Synthesis
Bromine (0.8 mL, 14.6 mmol) was added dropwise to a suspension of guanosine (4.0 g, 14.4 mmol) in water (100 mL), and the rate of addition was controlled so that the reaction mixture returned to colorless after each addition. The addition of bromine was terminated when the reaction mixture took on a yellowish color due to residual bromine. The colorless solid product was collected by rapid filtration and washed sequentially with 60 mL of cold water and 30 mL of cold acetone. The crude product was recrystallized by dissolving in 150 mL of hot water and subsequently dried under vacuum at 60 °C for 6 hours. Finally, 4.4 g (11.8 mmol, 85% yield) of the target compound 13 was obtained as colorless needle-like crystals with a melting point of 198-200 °C. The NMR data were as follows: 1H NMR (400 MHz, DMSO-d6) δ: 10.82 (1H, s), 6.50 (2H, s), 5.69 (1H, d, J = 6.4 Hz), 5.45 (1H, d, J = 6.0 Hz), 5.09 (1H, d, J = 5.2 Hz), 5.02 (1H, dd, J = 6.0, 11.6 Hz), 4.02 (1H, dd, J = 6.0, 11.6 Hz). 11.6 Hz), 4.92 (1H, t, J = 6.0 Hz), 4.14 (1H, m), 3.86 (1H, m), 3.66 (1H, m), 3.52 (1H, m); 13C NMR (100 MHz, DMSO-d6) δ: 155.5, 153.4, 152.0, 121.2, 117.5, 89.7, 85.8, 70.5, 70.3, 62.0.
References
[1] Photochemistry and Photobiology, 2018, vol. 94, # 4, p. 677 - 684
[2] Nucleosides, Nucleotides and Nucleic Acids, 2012, vol. 31, # 10, p. 707 - 719
[3] Indian Journal of Chemistry - Section B Organic and Medicinal Chemistry, 2001, vol. 40, # 5, p. 382 - 385
[4] Indian Journal of Chemistry - Section B Organic and Medicinal Chemistry, 2002, vol. 41, # 2, p. 379 - 383
[5] Tetrahedron Letters, 2016, vol. 57, # 27-28, p. 2949 - 2953