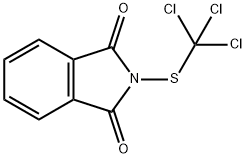
Description
Folpet is practically insoluble in water. It is a protective leaf fungicide. Its mode of action
inhibits normal cell division of a broad spectrum of microorganisms. It is used to control
cherry leaf spot, rose mildew, rose black spot, and apple scab. It is used on berries, flowers,
ornamentals, fruits, and vegetables and for seedand plant-bed treatment. It is also used
as a fungicide in paints and plastics and for treatment of internal and external structural
surfaces of buildings. It is incompatible with strongly alkaline preparations, such as
lime sulphur.
Chemical Properties
Off-White to Pale Yellow Solid
Uses
Folpet is a pesticide, fungicide agent from the
thiophtalimide group. Occupational exposure occurs
mostly in agricultural workers or in florists.
Uses
Agricultural fungicide.
Uses
Folpet is used to control downy mildews, powdery mildews, leaf
spot diseases, scab and rots in fruit, ornamentals and vegetables.
Definition
ChEBI: A member of the class of phthalimides that is phthalimide in which the hydrogen attached to the nitrogen is replaced by a trichloromethylthio group. An agricultural fungicide, it has been used to control mildew, leaf spot, and other diseases in crops sice
he 1950s.
General Description
White crystals. Used as a fungicide. Insoluble in water.
Air & Water Reactions
Insoluble in water. Hydrolyzed in alkaline solution. Hydrolysis products are corrosive to many metals.
Reactivity Profile
A halogenated phthalimide.
Contact allergens
Folpet is a pesticide, fungicide agent of thiophthalim ide group. Occupational exposure occurs mostly in
agricultural workers or in florists. Photosensitivity has
been reported.
Safety Profile
Moderately toxic by
ingestion. Questionable carcinogen with
experimental tumorigenic and teratogenic
data. Experimental reproductive effects.
Human mutation data reported. When
heated to decomposition it emits very toxic
fumes of Cl-, NOx, and SOx. Used as a
fungicide.
Carcinogenicity
An NCI bioassay of technicalgrade
captan was conducted to determine carcinogenicity by
administering captan in the feed to Osborne–Mendel rats and
B6C3F1 mice. The major outcome was that tumors of the
duodenum of B6C3F1 mice were associated with the captan
treatment. There was no evidence that the tumors observed in
Osborne–Mendel rats were treatment-related.
In the NCI study, groups of 50 rats of each sex were fed
average doses of 2520 or 6050 ppm captan in the diet for
80 weeks. Groups of 50 mice of each sex were fed 8000 or
16,000 ppm captan in the diet for 80 weeks. These doses are
approximately 250 (male) and 450 (female) mg/kg/day (high
dose) and 50 (male) to 100 (female) mg/kg/day (low dose) in
rats. In mice, these doses are approximately 2100 mg/kg/day
(high dose) and 1000 mg/kg/day (low dose).
Environmental Fate
Folpet rapidly degrades in both aquatic and terrestrial environments,
with a reported half-life ranging from 2.6 h to
2 days. The dissipation of folpet in the environment is
considered to be dependent on its hydrolysis in water and on
microbial-mediated degradation. Its rate of hydrolysis is greatly
influenced by pH, with more rapid hydrolysis observed at
higher, more alkaline pH levels.
Metabolic pathway
Folpet contains an unstable trichloromethylthio (sulfenyl) moiety that has
been shown to undergo rapid hydrolytic and metabolic degradation to
phthalimide (2). By analogy with captan, presumably the trichloromethylthio
moiety can be transferred to the sulfur atoms of thiols such
as cysteine and glutathione. Thus in the presence of thiols such as glutathione,
folpet is probably cleaved at the N-S bond to form thiophosgene
(3) and other gaseous products such as hydrogen sulfide, hydrogen
chloride and carbonyl sulfide. Thiophosgene (3) is rapidly hydrolysed by
water. The trichloromethylthio group and thiophosgene are believed to be
intermediates in the formation of thiazolidine-2-thione-4-carboxylica cid
(4) which is an addition product with cysteine. A thiazolidine derivative
of glutathione may also be formed (5). Folpet is metabolised in plants and
animals to phthalimide (2) and further to phthalamic acid (6) and phthalic
acid (7) (see Scheme 1).
Degradation
Folpet is hydrolysed rapidly in strongly alkaline conditions (PM). The
hydrolytic DT
50 of folpet is 1.1 hours at pH 7. The half-life for hydrolysis
of folpet in a commercial formulation was 12 hours at pH 7.35. Folpet
was decomposed in dilute, aqueous sulfuric acid with a half-life 10.5
hours at pH 3. The products were mainly phthalimide (2) and small
amounts of phthalamic acid (6) and phthalic acid (7). Phthalamic acid (6)
had completely degraded by the time all of the folpet had decomposed
(Cabras et al., 1997).
Folpet reacts with thiols in two steps. Firstly, phthalimide (2), thiophosgene
(3), hydrochloric acid and the corresponding disulfide are
produced. Secondly, depending on the thiol, thiophosgene (3) can react
with any remaining thiols to give trithiocarbonates, thiurams, etc. In
some cases as, for example, with L-cysteine, thiophosgene (3) combines
with amino and thiol groups of the reactant yielding the cyclic 2-
thiazolidinethiones (4) (Davidek and Seifert, 1975). Folpet reacts with
reduced glutathione (GSH) to produce mainly oxidised glutathione
(GSSG). Five unidentified products contained all or a portion of the
trichloromethyl moiety. Gaseous products including carbonyl sulfide
were released (Siegel, 1970).
Toxicity evaluation
Both folpet and its reactive metabolite, thiophosgene, interact
with thiol groups and denature proteins. This reaction is
responsible for its fungicidal/biocidal activity and its cellular
toxicity in mammals. Due to the toxicokinetics of this degradation,
folpet toxicity in mammals is generally limited to local
irritation effects at the site of contact.