Chemical Properties
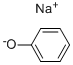
Sodium phenate, or sodium phenoxide, NaOC6H5, is a white crystalline substance that easily dissolves in water and alcohol. It decomposes when exposed to carbon dioxide in the air. It is produced by reacting sodium hydroxide solution (not carbonate) with phenol and subsequently evaporating the mixture. Sodium phenate is commonly used in the production of sodium salicylate.
Uses
Sodium phenoxide is used as a precursor to aryl ethers and involved in the preparation of phenyl ethers and metal phenolates. It serves as an antiseptic and used in organic synthesis.
Uses
As a general disinfectant, either in solution or mixed with slaked lime, etc., for toilets, stables, cesspools, floors, drains, etc.; for the manufacture of colorless or light-colored artificial resins, many medical and industrial organic Compounds and dyes; as a reagent in chemical analysis. Pharmaceutic aid (preservative).
Definition
ChEBI: Sodium phenolate is a phenolate. It has a role as a disinfectant.
Preparation
Sodium phenoxide was freshly prepared by adding sodium metal to phenol (0.45 g, 4.8 mmol) dissolved in diethyl ether (15 cm3), under an atmosphere of dry nitrogen. This was refluxed with (11) (1.0 g, 2.8 mmol) for 23 h. Following evaporation of the ether, water (20 cm3) was added and the mixture extracted into DCM. The DCM solution was dried (MgS04) and evaporated to give a solid (1.2 g). Fractional sublimation and recrystallisation from acetonitrile gave recovery of 2,4,6-tribromo-3,5-difluoropyridine (0.8 g, 80%) and 3-phenoxy-5-fluoro-2,4,6-tribromopyridine (0.2 g, 20%), m.p. 100±1 OC (Found: C, 31.3; H, 1.1; N, 2.9. C11H5Br3FNO requires C, 31.0; H, 1.2; N, 3.3%); IR spectrum no. 15; mass spectrum no. 15; nmr spectrum no. 19.
General Description
A white to reddish colored solid in the form of crystalline rods. Used as an antiseptic and in organic synthesis.
Air & Water Reactions
Decomposes in air. Soluble in water. In both cases, a corrosive alkaline solution forms that is a strong irritant to skin and eyes.
Reactivity Profile
Salts, basic, such as SODIUM PHENOLATE, are generally soluble in water. The resulting solutions contain moderate concentrations of hydroxide ions and have pH's greater than 7.0. They react as bases to neutralize acids. These neutralizations generate heat, but less or far less than is generated by neutralization of the bases in reactivity group 10 (Bases) and the neutralization of amines. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible.
Hazard
Strong irritant to skin and tissue.
Safety Profile
Poison by subcutaneous route. A corrosive irritant to skin, eyes, and mucous membranes. When heated to decomposition it emits toxic fumes of Na2O. See also PHENOL and SODIUM HYDROXIDE.
Purification Methods
The ground powder is washed with Et2O, then heated at 60o/1mm for 12 to 24hours to remove any free phenol and solvent. [Kornblum & Lurie J Am Chem Soc 81 2710 1959, Beilstein 6 I 718.]