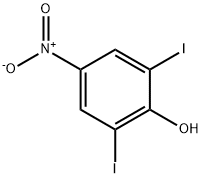
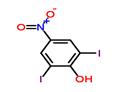
Chemical Properties
light yellow feathery crystals or yellow crystalline solid
Uses
2,6-Diiodo-4-nitrophenol is useful for statistical methods for developing QSARs to predict toxicity of phenols to Tetrahymena pyriformis.
Definition
ChEBI: Disophenol is a member of 4-nitrophenols.
General Description
Light yellow feathery crystals (from glacial acetic acid) or yellow crystalline solid Used against hookworm.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
2,6-Diiodo-4-nitrophenol is a halogenated and nitrated phenol. Reacts as a weak acid. Incompatible with strong reducing substances such as hydrides, nitrides, alkali metals, and sulfides. Flammable gas (H2) is often generated, and the heat of the reaction may ignite the gas. May generate heat with bases.
Health Hazard
ACUTE/CHRONIC HAZARDS: When heated to decomposition 2,6-Diiodo-4-nitrophenol emits very toxic fumes.
Fire Hazard
Flash point data for 2,6-Diiodo-4-nitrophenol are not available. 2,6-Diiodo-4-nitrophenol is probably combustible.
Safety Profile
Poison by ingestion,
intraperitoneal, subcutaneous, intravenous,
and parented routes. An anthelmintic.
When heated to decomposition it emits very
toxic fumes of Iand NO,. See also NITRO
COMPOUNDS of AROMATIC
HYDROCARBONS.
Toxicology
2,6-Diiodo-4-nitrophenol is toxic, with signs of toxicity in experimental animals (rodents and hounds) manifesting as increased respiration, heart rate and body temperature, as well as very rapid rigours. The increased toxicity after repeated administration is related to the accumulation of 2,6-Diiodo-4-nitrophenol in the plasma
[1].
References
[1] KAISER J A. Studies on the toxicity of disophenol (2,6-diiodo-4-nitrophenol) to dogs and rodents plus some comparisons with 2,4-dinitrophenol[J]. Toxicology and applied pharmacology, 1964. DOI:10.1016/0041-008X(64)90108-5.