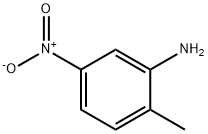
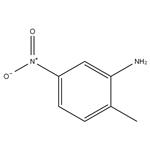
Description
2-Methyl-5-nitroaniline, also known as 5-nitro-o-toluidine, is an intermediate compound in the synthesis of a wide range of azo dyes and is an in vivo metabolic product of 2,4-dinitrotoluene.

Chemical Properties
5-Nitro-o-toluidine is a yellow, crystalline
solid.
Uses
5-Nitro-o-toluidine is used in the synthesis of numerous dyes.
Uses
2-Methyl-5-nitroaniline may be employed as analytical standard for the surfactant mediated transformations (?254nm) of 2,4-dinitrotoluene.
Preparation
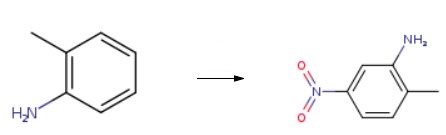
o-Toluidine(500 mg, 4.19 mmol) was added with vigorous stirring to a balloon with concentrated sulfuric acid (3.9 ml, 71.4 mmol). The temperature of the reaction is 10 ℃ (salt/ice, cold bath). After that, an acid mixture (0.9 mlHNO3 and 0.9 ml H2SO4) was added to the solution for 2 h. The mixture was placed on ice and basified with NaOH. Finally, 2-methyl-5-nitroaniline was collected by filtration and washed with water.
Definition
ChEBI: A C-nitro compound in which the nitro compound is meta to the amino group and para to the methyl group of o-toluidine.
Preparation
2-Methyl-5-nitrobenzenamine?in concentrated sulfuric acid nitration.
Air & Water Reactions
Insoluble in water. 2-Methyl-5-nitroaniline is sensitive to moisture, light, or prolonged exposure to air.
Reactivity Profile
2-Methyl-5-nitroaniline is incompatible with strong oxidizing agents, acids, acid chlorides, acid anhydrides and chloroformates.
Fire Hazard
Flash point data for 2-Methyl-5-nitroaniline are not available; however, 2-Methyl-5-nitroaniline is probably combustible.
Toxicology
Genetic toxicity studies for 2-methyl-5-nitroaniline indicate generally positive results in
reverse-mutation assays in Salmonella typhimurium and Escherichia coli, and in Syrian hamster
embryo (SHE) cell transformation assays; the compound is weaker in potency than other 2,4-dinitrotoluene
metabolites.
Safety Profile
Confirmed carcinogen
with experimental carcinogenic data.
Moderately toxic by ingestion. Mutation data
reported. Decomposes exothermically when
heated to 150℃. When heated to
decomposition it emits toxic fumes of NOx.
See also NITRO COMPOUNDS OF
AROMATIC HYDROCARBONS.
Carcinogenicity
There
are no data available for evaluating carcinogenic risk to
humans. When 5-nitro-o-toluidine was administered in a
dietary feeding study to F344 rats (50 or 100 ppm) and
B6C3F1 mice (1200 or 2300 ppm) of both sexes, hepatocellular
carcinomas were produced in mice but not in rats.
Shipping
UN2660 Nitrotoluidines (mono), Hazard Class:
6.1; Labels: 6.1-Poisonous materials.
Purification Methods
Acetylate the aniline, and the acetyl derivative is crystallised to constant melting point; then hydrolyse it with 70% H2SO4 and the free base is regenerated by treatment with NH3 [Bevan et al. J Chem Soc 4284 1956]. [Beilstein 12 H 844. 12 IV 1807.]
Incompatibilities
Incompatible with oxidizers (chlorates,
nitrates, peroxides, permanganates, perchlorates, chlorine,
bromine, fluorine, etc.); contact may cause fires or explosions.
Keep away from alkaline materials, strong bases,
strong acids, oxoacids, epoxides, acid chlorides; acid anhydrides;
chloroformates.
Waste Disposal
Consult with environmental
regulatory agencies for guidance on acceptable disposal
practices. Generators of waste containing this contaminant
(≥100 kg/mo) must conform with EPA regulations governing
storage, transportation, treatment, and waste disposal.