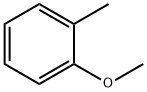
Chemical Properties
o-Methylanisole has a pungent, warm, floral odor with earthy, walnut undertones. It has a sweet, fruity, nut-like flavor
at low levels.
Physical properties
clear colorless to light yellow liquid. soluble in alcohol and ether, insoluble in water.
Occurrence
Reported found in starfruit, mastic gum oil and rooibus tea (Aspalathus linearis).
Uses
The catalytic system of disubstituted aromatics was optimized for the 2-methylanisole reduction by a proper choice of the amine/Rh ratio which should be high enough to stabilize very small colloidal rhodium particles and low enough to avoid deactivation. The total synthesis of (±)-heliannuol D and its epimer has been completed in 9 steps and 12% overall yield from 2-methylanisole. The thermal activation of 2-methylanisole (60°C) by the Ir (III) complex TpMe2Ir (C6H5) 2 (N2)(1; TpMe2= hydrotris (3, 5-dimethylpyrazolyl) borate) yielded a mixture of hydride complexes. The total synthesis of the phenolic sesquiterpene mutisianthol has been accomplished in 12 steps from the readily available 2-methylanisole. The catalytic system was optimized for the 2-methylanisole reduction by a proper choice of the amine/Rh ratio which should be high enough to stabilize very small colloidal rhodium particles and low enough to avoid deactivation.
Definition
ChEBI: 2-methylanisole is a monomethoxybenzene that is o-cresol in which phenolic hydroxy group has been converted to the corresponding methyl ether. A 'green' solvent (b.p. 171°C) and food flavour ingredient, it is found in mastic oils, virgin olive oils and frankincense. It has a role as a polar aprotic solvent and a flavouring agent. It is a monomethoxybenzene, a volatile organic compound and a member of toluenes. It derives from an o-cresol.
Preparation
2-Methylanisole is synthesized by methylation of o-cresol using dimethylsulfate in caustic soda at 40°C.
synthesis of 2-methylanisole: Make sodium hydroxide into a 20% solution, stir and mix with o-cresol, cool to below 10°C, and slowly add dimethyl sulfate dropwise. After the addition was completed, the temperature was raised to 40 °C for 20 min, and then reacted with 100 °C for 12 h. Then the reactant was washed with water until neutral, water was removed, distilled, and the fraction at 171°C was collected to obtain the finished product of 2-methylanisole.
Aroma threshold values
Detection: 600 ppb. Aroma characteristics at 1.0%: naphthyl, camphoreous, phenolic and woody with a
salicylate nuance.
Taste threshold values
Taste characteristics at 5.0 ppm: camphoreous, earthy, woody and alicylate with minty, spicy nuances.
General Description
2-Methylanisole is found in mastic oils, virgin olive oils and frankincense. It is a monomethoxybenzene and acts as an intermediate for the preparation of compounds with methylhydroquinone core.
Flammability and Explosibility
Flammable