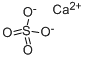Description
Calcium sulfate and its hydrates are important industrial compounds that have been used throughout history. Calcium sulfate is obtained naturally from mined gypsum rock, but it also exists in mineral form. Gypsum forms in beds as sedimentary rock when calcium sulfate, which is a natural component of seawater, is deposited as shallow marine water bodies evaporate. Gypsum is a transparent, soft, white mineral and is the most common sulfate mineral.
Chemical Properties
white powder or granules
Chemical Properties
Both calcium sulfate and calcium sulfate dihydrate are white or offwhite,
fine, odorless, and tasteless powder or granules.
Chemical Properties
Calcium sulfate forms white to clear crystals.
It is commonly encountered in the anhydrous form or as
the dihydrate.
Physical properties
Anhydrous calcium sulfate is a crystalline substance; orthorhombic; the color may vary as white, gray, blue or brick-red; occurs as insoluble anhydrite or porous soluble anhydrite; density 2.96 g/cm
3; hardness 3.5 Mohs; insoluble anhydrite is practically insoluble in water (0.21% at 20°C); soluble anhydrite readily absorbs moisture and is soluble in water.
Hemihydrate is a white fine powder; sparingly soluble in water (3g/L at 25°C); combines with water, setting to a hard mass.
Dihydrate may occur as lumps or powder; density 2.32 g/cm
3; partially loses water on heating at 100°C; slightly soluble in water (2.4 g/L at 25°C); KSP =2.4x10-
5; almost insoluble in organic solvents.
History
Gypsum’s use dates back to prehistoric times. Archeological evidence shows that it was mined from caves and used to paint ancient gravestones. The earliest evidence of its use as a building material dates from 6000 b.c.e. in the southwest Asian areas of ancient Anatolia and Syria. Egyptians used gypsum and plaster in their buildings and monuments, with both found in the Great Pyramids built around 3700 b.c.e. Calcium sulfate has been used for more than 2,000 years in China to produce tofu. The word gypsum comes from the Greek word for chalk, gypsos. The Greek natural philosopher Theophrastus (371–286 b.c.e.) referred to gypsum in his writings. Gypsum was extensively used in Roman times and throughout the Middle Ages.
In the 1700s, Paris was a leading plaster center, with most of its buildings made using plaster. After the fire of London in 1666 destroyed 80% of the city, the king of France ordered all wooden houses in France to be covered with plaster as protection against fire. The hemihydrate form got the name plaster of Paris from the extensive gypsum deposits quarried in the Montmartre district of Paris. In 1765 and 1766, Antoine Lavoisier (1743–1794) presented papers to the French Academy of Science on gypsum that explained the setting of plaster. Lavoisier determined that gypsum is a hydrated salt and that set plaster occurs when the hemihydrate form rehydrates back to gypsum.
Uses
The largest use of calcium sulfate is in the construction industry, which accounts for more than 90% of its production.Gypsum has hundreds of other applications outside the construction industry. It can be used agriculturally for several purposes: to supply calcium and sulfur to soils, to balance pH, and to condition soil. Food grade calcium sulfate is used as a calcium supplement in enriched foods such as flour, cereals, and baked goods. It is used as a gelling and firming agent with canned vegetables. Calcium sulfate is the most common tofu coagulant. The positively charged calcium ion in calcium sulfate attracts the negatively charged groups in protein molecules, causing thermally denatured proteins to coagulate. Anhydrous calcium sulfate is used as a filler to whiten food and consumer products such as frostings, ice creams, paper, paints, and toothpaste. Powdered gypsum can be used as a chalk for marking athletic fields and other large areas.
Uses
The insoluble anhydrite is used in
cement formulations and as a paper filler; the
soluble anhydrite is used as a drying agent; the
hemihydrate is used for wall plaster and wallboard;
gypsum is used in manufacture of
plaster of paris and portland cement.
Uses
Pharmaceutic
aid (tablet and capsule diluent).
Production Methods
Anhydrous calcium sulfate occurs naturally as the mineral
anhydrite. The naturally occurring rock gypsum may be crushed
and ground for use as the dihydrate or calcined at 1508℃ to produce
the hemihydrate. A purer variety of calcium sulfate may also be
obtained chemically by reacting calcium carbonate with sulfuric
acid or by precipitation from calcium chloride and a soluble sulfate.
Production Methods
Gypsum is mined throughout the world, with gypsum mines in 90 countries. In addition to mined gypsum, gypsum is produced synthetically as a by-product of chemical processes. Most synthetic gypsum comes from flue gas desulfurization used to control sulfur emissions from electric power generation, especially coal-burning power plants. In this process calcium carbonate reacts with sulfur dioxide to produce calcium sulfite (CaSO
3): CaCO
3(s) + SO
2(g) → CaSO
3(s) + CO
2(g). Calcium sulfite is then oxidized to gypsum: 2CaSO
3(s) + O
2(g) + 4H
2O
(l) → 2CaSO
4?2H
2O
(s). The second major source of synthetic gypsum is acid neutralization. The sulfate production of titanium dioxide, TiO
2, used as a whitener in many commercial products, yields gypsum as a by-product in the process of neutralizing acidic waste. Gypsum is also generated from sugar and citric acid production. In the latter, dilute citric acid (C
6H
8O
7) is precipitated with calcium hydroxide as calcium citrate (Ca
3(C
6H
5O
7)
2).
Definition
anhydrite: An important rockforminganhydrous mineral form ofcalcium sulphate, CaSO
4. It is chemicallysimilar to gypsum but isharder and heavier and crystallizes inthe rhombic form (gypsum is monoclinic).Under natural conditions anhydriteslowly hydrates to formgypsum. It occurs chiefly in whiteand greyish granular masses and isoften found in the caprock of certainsalt domes. It is used as a raw materialin the chemical industry and inthe manufacture of cement and fertilizers.
General Description
Odorless, white powder or colorless, crystalline solid. Crystals sometimes have a blue, gray or reddish tinge or can be brick red. Density: 2.96 g cm-3.
Reactivity Profile
CALCIUM SULFATE is non-combustible. Decomposes to give toxic oxides of sulfur, but only at very high temperature (>1500°C). Generally of low reactivity but may act as an oxidizing agent: incompatible with diazomethane, aluminum, and phosphorus. Certain forms of CALCIUM SULFATE react with water; others do not. INSOLUBLE ANHYDRITE or dead-burned gypsum is made by the dehydration of CALCIUM SULFATE dihydrate (gypsum) at high (> 600°C) temperature. At room temperature, insoluble anhydrite dissolves very slowly to the extent of 0.24 g per 100 g of water and does not absorb moisture from the air. SOLUBLE ANHYDRITE, which is obtained by heating CALCIUM SULFATE dihydrate at a temperature below 300°C, has a high affinity for water and is used as a desiccant. Soluble anhydrite absorbs water to form CALCIUM SULFATE hemihydrate (Plaster of Paris).
Health Hazard
Calcium sulfate is considered to
be a nuisance dust.
There have been no reports of adverse
effects in humans exposed to calcium sulfate.
Excessive concentrations would be expected to
cause reduced visibility and skin and upper
respiratory tract irritation.1 One report on
gypsum miners attributed adverse effects to
respirable quartz rather than calcium
sulfate.
Agricultural Uses
Anhydrite is a naturally occurring, solid, white mineral
called anhydrous calcium sulphate (CaSO
4). It differs
from gypsum in hardness and in hydration. It is used as a
raw material in the chemical industry and in the
manufacture of fertilizers and cement.
Pharmaceutical Applications
Calcium sulfate dihydrate is used in the formulation of tablets and
capsules. In granular form it has good compaction properties and
moderate disintegration properties.
Calcium sulfate hemihydrate, is used in the
preparation of plaster of Paris bandage, which is used for the
immobilization of limbs and fractures; it should not be used in the
formulation of tablets or capsules.
Anhydrous calcium sulfate is hygroscopic and is used as a
desiccant. The uptake of water can cause the tablets to become very
hard and to fail to disintegrate on storage. Therefore, anhydrous
calcium sulfate is not recommended for the formulation of tablets,
capsules, or powders for oral administration.
Therapeutically, calcium sulfate is used in dental and craniofacial
surgical procedures.
Safety Profile
A nuisance dust. Reacts
violently with aluminum when heated.
Mixtures with diazomethane react
exothermically and eventually explode.
Mixtures with phosphorus ignite at high
temperatures. When heated to
decomposition it emits toxic fumes of SO,,
See also CALCIUM COMPOUNDS and
SULFATES.
Safety
Calcium sulfate dihydrate is used as an excipient in oral capsule and
tablet formulations. At the levels at which it is used as an excipient,
it is generally regarded as nontoxic. However, ingestion of a
sufficiently large quantity can result in obstruction of the upper
intestinal tract after absorption of moisture.
Owing to the limited intestinal absorption of calcium from its
salts, hypercalcemia cannot be induced even after the ingestion of
massive oral doses.
Calcium salts are soluble in bronchial fluid. Pure salts do not
induce pneumoconiosis.
Potential Exposure
Calcium sulfate is used as a pigment;
in Portland cement, in tiles and plaster; in polishing powders, a filler in paints and paper coatings; in the drying of
gases and liquids; a soil conditioner; in molds and surgical
casts; in wallboard, and many others.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately.If this chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.
storage
Calcium sulfate is chemically stable. Anhydrous calcium sulfate is
hygroscopic and may cake on storage. Store in a well-closed
container in a dry place, avoiding heat.
Shipping
Calcium sulfate is a “NONREGULATED
MATERIAL.”
Incompatibilities
In the presence of moisture, calcium salts may be incompatible with
amines, amino acids, peptides, and proteins, which may form
complexes. Calcium salts will interfere with the bioavailability of
tetracycline antibiotics. It is also anticipated that calcium sulfate
would be incompatible with indomethacin, aspirin, aspartame, ampicillin, cephalexin, and erythromycin since
these materials are incompatible with other calcium salts.
Calcium sulfate may react violently, at high temperatures, with
phosphorus and aluminum powder; it can react violently with
diazomethane.
Incompatibilities
Contact with diazomethane, aluminum,
phosphorus, and water may cause explosions. Note:
Hygroscopic material (i.e., absorbs moisture from the air).
Reacts with water, forming gypsum and plaster of Paris.
Waste Disposal
Landfilling
Regulatory Status
GRAS listed. Accepted for use as a food additive in Europe.
Included in the FDA Inactive Ingredients Database (oral capsules,
sustained release, tablets). Included in nonparenteral medicines
licensed in the UK and Europe. Included in the Canadian List of
Acceptable Non-medicinal Ingredients.