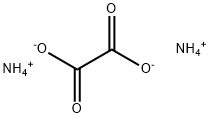
Description
Ammonium oxalate, C
2H
8N
2O
4 (some times written as (NH
4)
2C
2O
4), is an oxalate salt with ammonium (sometimes as a monohydrate). It is a constituent of some types of kidney stone. Found also in guano.
Chemical Properties
Ammonium oxalate is an odorless, colorless
crystalline material or powder.
Uses
Ammonium oxalate is used as an analytical reagent and general reducing agent. It is commonly employed in soil chemical analysis to extract iron and aluminum from poorly-crystal line minerals.
Definition
ChEBI: An ammonium salt consisting of ammonium and oxalate ions in a 2:1 ratio.
Flammability and Explosibility
Not classified
Safety Profile
A poison. Can react
violently with (NaOCl+ ammonium
acetate). When heated to decomposition it
SYNS: ETHANEDIOIC ACID DIAMMONIUN SALT 0
OXALIC ACID, DIAIMMONIUM SALT
can emit toxic fumes of NH3 and NOx. See
also OXALATES.
Potential Exposure
It is used in chemical analysis and to
make blueprint paper, explosives; a rust-removal ingredient
in metal polishes.
Shipping
UN2811 Toxic solids, organic, n.o.s., Hazard
Class: 6.1; Labels: 6.1-Poisonous materials, Technical
Name Required. UN1759 Corrosive solids, n.o.s., Hazard
class: 8; Labels: 8-Corrosive material, Technical Name
Required.
Incompatibilities
Ammonium oxalate is a reducing agent
and also reacts as a base to neutralize acids and reacts with
oxidizers generating carbon dioxide. Incompatible with oxidizers (chlorates, hypochlorite solutions, nitrates, peroxides,
permanganates, perchlorates, chlorine, bromine, fluorine,
etc.); contact may cause fires or explosions. Keep
away from alkaline materials, strong bases, strong acids,
oxoacids, epoxides.